Salivary gland carcinosarcoma is an exceedingly rare and aggressive neoplasm composed of a mixture of carcinomatous and sarcomatous components, with either component capable of metastasis. Salivary gland carcinosarcoma can arise in pleomorphic adenoma (PA) or de novo.1
The present study reports a case of the parotid gland de novo carcinosarcoma, which contained salivary duct adenocarcinoma and sarcoma that resembled malignant fibrous histiocytoma (MFH). To the authors’ knowledge, MFH as a component of salivary gland carcinosarcoma has been described only once in literature, and was combined with squamous cell carcinoma.2
Case presentationA 45-year-old man presented with a mass in the right parotid gland that had been rapidly enlarging for eight weeks. Preoperative ultrasound showed a tumor in the parotid gland but without sign of metastasis in regional lymph nodes. The patient underwent right total parotidectomy, and gross inspection revealed a relatively well-defined, gray-white, homogeneous, solid tumor that measured 2.5cm in the largest diameter.
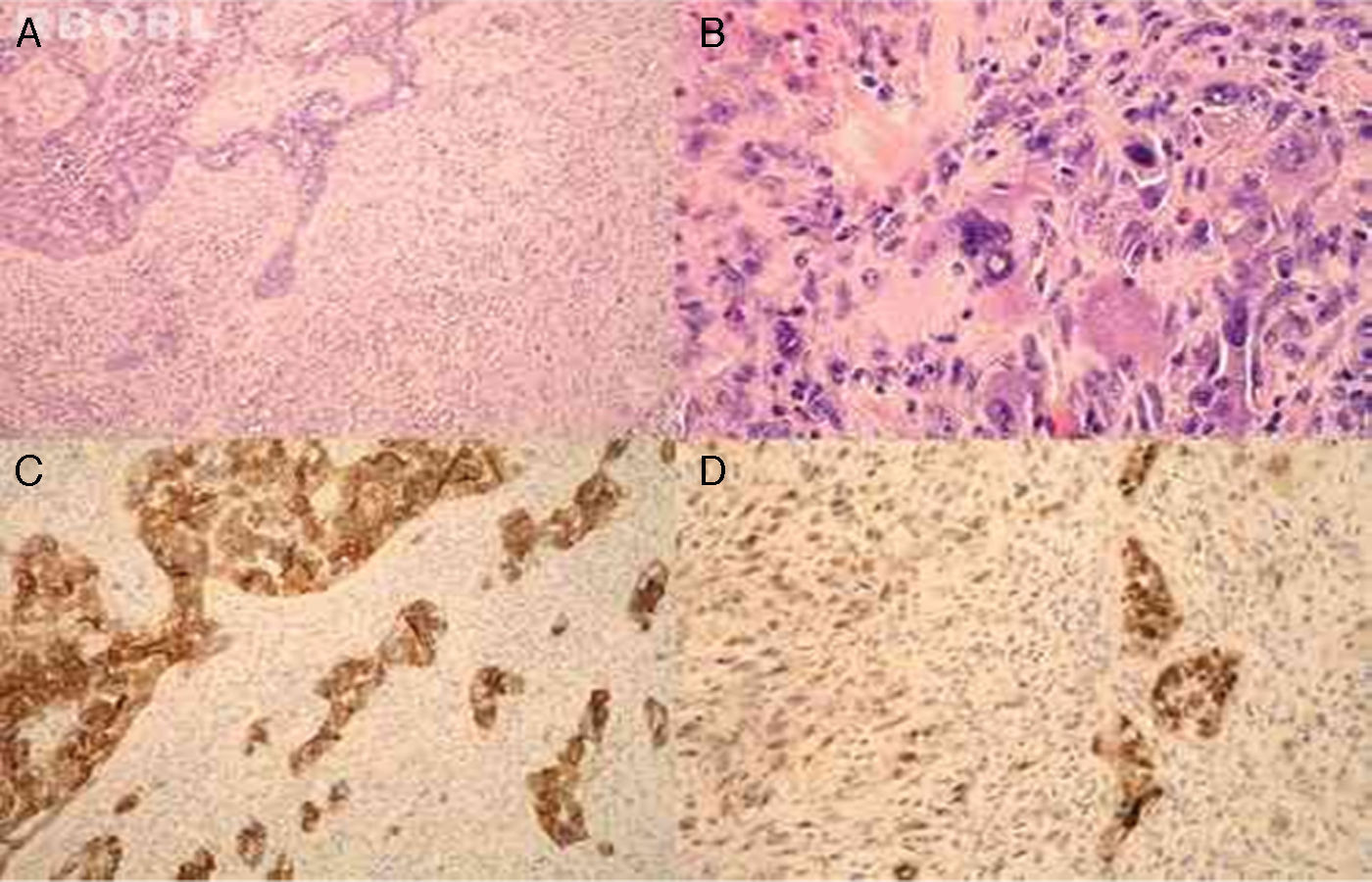
Microscopically, the tumor was composed of two malignant components, carcinoma and sarcoma (Fig. 1A). The former consisted of salivary duct adenocarcinoma with typical foci of comedonecrosis. The latter was composed of markedly atypical, large polygonal cells with abundant eosinophilic, focally foamy cytoplasm, with multilobated to multiple nuclei, admixed with a population of spindle cells arranged in short fascicles. Mitotic figures were readily identified; some were atypical. Thus, the mesenchymal component of the tumor histologically corresponded to MFH (Fig. 1B). Immunohistochemistry showed that the carcinomatous component was positive for cytokeratin AE1/AE3, cytokeratin 7, CEA, and HER2 (Fig. 1C). The sarcomatous component was diffusely positive for vimentin. p53 showed focal, nuclear positivity in both malignant components (Fig. 1D).
The tumor was composed of two different malignant components, carcinoma (left) and sarcoma (right) (A, ×100, HE). The sarcomatous component morphologically corresponded to malignant fibrous histiocytoma (B, ×400, HE). Immunohistochemically, the carcinoma was positive for HER2 (C, ×100, HER2). p53 showed focal, nuclear positivity in both malignant components (D, ×200, p53).
The multiple sections showed no evidence of a pre-existing PA, and diagnosis of de novo carcinosarcoma of the parotid gland composed of salivary duct adenocarcinoma and sarcoma that resembled MFH was established.
DiscussionSalivary gland carcinosarcomas are rare, and therefore a well established therapeutic approach is lacking. In some cases, patients were treated only with surgery, but the most common treatment includes a combination of surgery and radiotherapy. Despite aggressive therapy, local recurrence and metastases occur frequently; the mean survival is approximately 19 months.1
The present patient was additionally treated with radiotherapy (48Gy in 16 fractions, twice a day); nine months after surgery, the patient showed no sign of local recurrence or distant metastasis.
It is still unclear whether the two malignant components in carcinosarcoma of salivary glands occur by collision of two independent tumors, or they are of clonal origin. The first studies based on immunohistochemical analysis indicated that the distinct cellular entities reflect two independent neoplastic processes.2,3 More recent molecular studies demonstrated that the two histologically different components would result from a dichotomous differentiation pattern of a common stem cell, or by trans-differentiation from one cell type to another.4–6 Fowler et al.4 tested loss-of-heterozygosity rates at loci of known tumor suppressors in carcinosarcomas and found a 73% agreement in the mutational profiles of the two components. Vékony et al.,5 using comparative genomic hybridization, observed a 75% homology between the DNA copy numbers in epithelial and mesenchymal components of parotid gland carcinosarcoma. Furthermore, Völker et al.6 demonstrated the losses on chromosome 13 in PA and the sarcomatous component of carcinosarcoma, and concluded that this could represent an early event in differentiation of a common pluripotent stem cell line
Final remarksIn the present case, p53 expression in carcinomatous and sarcomatous cells could indicate identical early events in the carcinogenesis of both malignant components. Therefore, despite morphological and immunohistochemical differences between epithelial and mesenchymal components, the authors believe that both elements of carcinosarcoma have evolved from a single common precursor cell, probably a myoepithelial cell.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Tomas D, Vagic D, Bedekovic V, Kruslin B. Carcinosarcoma de novo of the parotid gland with unusual sarcomatous component. Braz J Otorhinolaryngol. 2014;80:364–5.