Squamous cell carcinoma is the most common neoplasm of the larynx, and its evolution depends on tumor staging. Vascular endothelial growth factor is a marker of angiogenesis, and its expression may be related to increased tumor aggressiveness, as evidenced by the presence of cervical lymphatic metastases.
ObjectivesTo evaluate the expression of the vascular endothelial growth factor marker in non-glottic advanced squamous cell carcinoma of the larynx (T3/T4) and correlate it with the presence of cervical lymph node metastases.
MethodsRetrospective clinical study and immunohistochemical analysis of vascular endothelial growth factor through the German scale of immunoreactivity in products of non-glottic squamous cell carcinomas.
ResultsThis study analyzed 15 cases of advanced non-glottic laryngeal tumors (T3/T4), four of which exhibited cervical lymphatic metastases. There was no correlation between vascular endothelial growth factor expression and the presence of cervical metastases.
ConclusionAlthough vascular endothelial growth factor was expressed in a few cases, there was no correlation with the spread of cervical lymph metastases.
O carcinoma de células escamosas é a neoplasia mais frequente da laringe e seu prognóstico depende do estadiamento. A progressão da doença está relacionada a fatores intrínsecos celulares do câncer, não conhecidos. O VEGF (vascular endothelial growth factor) é um marcador de angiogênese e sua expressão pode estar relacionada a uma maior agressividade tumoral, evidenciada pela presença de metástases linfáticas cervicais.
ObjetivosAvaliar a expressão do marcador VEGF em carcinoma de células escamosas da laringe avançados (T3/T4), não glóticos e correlacionar quanto à presença de metástases linfáticas cervicais.
MétodoEstudo clínico retrospectivo de análise imunohistoquimica do VEGF através da escala Germânica de imunorreatividade em produtos de carcinomas epidermóides não glóticos.
ResultadosAnalisados 15 casos de tumores avançados de laringe (T3/T4) não glóticos, sendo sete com presença de metástases linfáticas cervicais. Não houve correlação entre a expressão do VEGF e a presença de metástases cervicais.
ConclusãoO VEGF foi pouco expressado nos casos estudados e não foi observada sua correlação com a disseminação de metástase linfática cervical.
Angiogenesis is characterized by the formation of new capillary blood vessels that originate from a pre-existing vasculature. This process is essential to provide nutrients, oxygen, and growth factors that support cell function and survival. Therefore, it is associated with tumor growth and metastasis.1,2
A complex interaction between endothelial cells, extracellular protein matrix, and soluble factors in plasma occurs in angiogenesis. Endothelial cells leave their quiescent state when stimulated by VEGF (vascular endothelial growth factor) and initiate the following steps: dissolution of the basal membrane; migration and proliferation of endothelial cells; capillary tube formation; and maturation and survival of newly formed vessels. This ensures the required blood supply for neoplastic evolution.1,2
Squamous cell carcinoma is the most common neoplasm of the larynx and its prognosis depends on the size of the lesion, the level of local invasion, cervical lymphatic spread, and presence of distant metastases.1,3
Histologically, squamous cell carcinoma of the larynx and hypopharynx are similar to that of other regions and are classified into different degrees of differentiation by the neoplastic proliferation of squamous cells and the nature of tumor infiltration. Depending on the degree of keratinization and cellular atypia, they are classified as well, moderately, or poorly differentiated.3
Lymphatic drainage of the glottic region is scarce; this decreases the metastatic potential of tumors that develop in this region. The supraglottic and subglottic regions have rich lymphatic drainage, with the supraglottic region draining mainly into the upper jugulocarotid chain lymph nodes and to a lesser degree into the middle jugulocarotid chain lymph nodes whereas the subglottic region tends to drain ipsilaterally to the middle and lower jugulocarotid lymph nodes, that may drain to the anterosuperior mediastinal chain.3 For this reason, we decided to exclude glottal tumors from the study.
The literature discussing treatment of these tumors has increased and studies of new chemotherapeutic agents and targeted therapies have gained prominence. Examples of this include the promising results reported for cetuximab, a monoclonal antibody that blocks endothelial growth factor receptor (EGFR) used for the treatment of head and neck cancer, and bevacizumab and other recombinant anti-VEGF monoclonal antibodies.4,5
These antibodies bind to VEGF isoforms with high affinity and prevent the cytokine from binding with the endothelial cell receptor and triggering the angiogenesis process. The use of angiogenesis-inhibiting monoclonal antibodies for the treatment of colorectal, kidney, and lung cancer has already been approved by the FDA. For the treatment of larynx/head and neck cancer, they are still being assessed.4,5
Considering that VEGF plays an important role in tumor angiogenesis and that this factor is associated with tumor formation, progression, and metastasis, its expression could be related to increased tumor aggressiveness evidenced by cervical metastatic spread.2
The present study aimed to evaluate the expression of VEGF marker in advanced, non-glottic squamous cell carcinoma of the larynx (T3/T4) and correlate it with the presence or absence of cervical lymph node metastasis.
MethodsThis was a retrospective clinical study, conducted in a sample obtained by a cross-section design in a historical cohort, using materials (surgical specimens) obtained from the resection of non-glottic squamous cell carcinoma of the larynx from surgeries in a tertiary university hospital during a specified period of ten years. The procedures performed with the patients were hemi-laryngectomy or total laryngectomy, with respective lymphadenectomy.
Patients were divided by the presence or absence of metastases and by surgical-clinical staging. Cases of non-glottic squamous cell carcinoma of the larynx and hypopharynx were obtained from the tumor bank of the Pathological Anatomy Department of a tertiary hospital. Of these, 15 cases of advanced non-glottic larynx tumors were selected, as well as three cases of tumors of the base of the tongue, ten cases of supraglottic tumors, and two cases of hypopharynx/pyriform sinus tumors.
Inclusion and exclusion criteriaTumors whose paraffin blocks were located and contained enough material for new sections were included in the survey. We required adequate tissue and data for staging each lesion by its tumor location, and determining lesion size, level of invasion into adjacent tissues, lymph node involvement, and distant metastasis. Clinical data and tumor staging were obtained from patients’ records.
All cases that were not in accordance with the abovementioned criteria were excluded.
Immunohistochemical analysisImmunohistochemical analysis was carried out using the avidin-biotin-peroxidase method and the VEGF antibody (type IgG1, 1:100 dilution, Santa Cruz, pretreated with citrate buffer).
Positive and negative marker controls were prepared in order to compare with the studied cases. Lymphoid tissue was used as positive control (as per manufacturer's instructions).
Slide reading was carried out randomly, by an investigator who neither had knowledge of the previous diagnosis given by the pathologist nor knowledge of the patient's clinical status, by comparing the slides with positive and negative controls. Scanned photographs at high magnification (400×) were made of highly reactive areas (hot spots), using a Nikon COOLPIX Camera 995. After that, the images were sent to the computer for histological analysis, which used the Imagelab 2000 software.
All parameters were evaluated in a blinded manner.
Finally, the histological and immunohistochemical findings were correlated with the TNM stage (TNM-UICC-AJCC Classification [2010]).6
The degree of VEGF expression was based on the German scale of immunoreactivity. This consists in multiplying the coefficient of the immunomarker expression intensity by the percentage coefficient of positive cells.
The coefficient of the immunomarker expression intensity was graded from 0 to 3, thus characterized: 0, no staining; 1, stained weakly; 2, stained moderately; 3, stained strongly.
To obtain the percentage coefficient of positive cells, the following sequence was used: coefficient, represented by the number of positive (stained) and negative (not stained) cells, was counted on a sample space of at least 500 cells, and stratified as follows: coefficient of 0 if there was no staining; coefficient of 1 if there was 1–10% positivity; coefficient of 2 if there was 11–50% positivity; coefficient of 3 if there was 51–80% positivity; and coefficient of 4 if there was 81–100% positivity.
The final value of the German scale of immunoreactivity (coefficient of the immunomarker expression intensity×coefficient of positive cell percentage) ranged from 0 to 12, stratified as: 0 (negative), 1–4 (poor), 5–8 (moderate), and 9–12 (strong). To facilitate the analysis, the negative/weak cases were considered as group 1 and the moderate/strong cases as group 2.
Statistical analysisAfter pooling the data, statistical analyses were performed using the IBM software SPSS Statistics®, using the chi-squared test and Fisher's exact test.
To simplify and better analyze the data, they were grouped as follows:
- •
German Scale of Immunoreactivity:
Negative and weak – Group 1
Moderate and strong – Group 2
- •
Cell proliferation index:
Low and slight cell proliferation – Group 1
Moderate and high cell proliferation – Group 2
- •
TNM (TNM-UICC-AJCC Classification–2010)6
T1 and T2 – Group 1/T3 and T4 – Group 2
N0 – Group 0/N1, N2, and N3 – Group 1
The study was approved by the Ethics Committee of the institution (485/11).
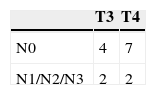
ResultsA total of 15 cases were evaluated, including four with cervical metastases.
The staging of these tumors, defined through TNM (2010), is described in Table 1. It can be observed that only four of the 15 cases had cervical metastases. Table 1 further details the distribution of the four cases with cervical metastasis according to the T classification.
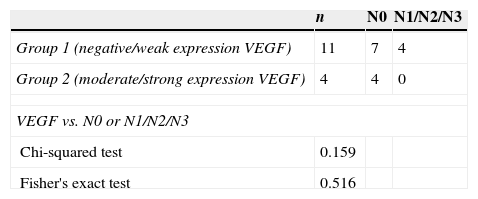
Table 2 shows the expression of VEGF in the studied cases, considering cases that had negative or weak expression as group 1, and moderate or strong expression as group 2 according to the German scale. Overexpression of VEGF in epithelial cells of squamous carcinomas was observed in only 26.6% of the cases studied.
Distribution of groups 1 and 2 (VEGF expression) in 15 cases, their correlation with the presence of cervical metastases, and statistical analysis.
| n | N0 | N1/N2/N3 | |
|---|---|---|---|
| Group 1 (negative/weak expression VEGF) | 11 | 7 | 4 |
| Group 2 (moderate/strong expression VEGF) | 4 | 4 | 0 |
| VEGF vs. N0 or N1/N2/N3 | |||
| Chi-squared test | 0.159 | ||
| Fisher's exact test | 0.516 | ||
VEGF, vascular endothelial growth factor.
Table 2 also shows the correlation of VEGF expression in groups 1 and 2, according to the presence or absence of cervical lymph node metastasis, with p=0.363.
There was no correlation between the overexpression of this marker with the presence of cervical lymph node metastasis (p=0.159).
DiscussionIn the literature, studies evaluating head and neck tumors, which include not only squamous cell carcinomas of the larynx (glottic and non-glottic), but also oral cavity and pharynx tumors, have reported an increase in VEGF correlated with increased angiogenesis, with neoplastic progression, and with prognosis; the analyses took into account the positivity of the marker, regardless of its intensity.1,2,7–9 There is only one recent study that specifically addressed laryngeal cancer and its association with VEGF. In this case, the positivity of this marker was higher than 72%,10 unlike the results found here, perhaps due to the small number of cases in the present sample.
There is divergence among authors regarding the correlation between VEGF expression and the presence of lymph node and distant metastases. Some authors found positive results between VEGF expression and the presence of cervical lymph node metastases and distant metastases.2,8,9,11
The meta-analysis by Panayiotis et al. found that VEGF correlates with poor survival in patients with head and neck squamous cell carcinoma; however, the correlation of VEGF overexpression with the presence of lymph node metastasis/distant metastasis is yet to be established.12 Boonkitticharoen also found no correlation between this marker and metastases.13
A recent Asian study reported a close association between VEGF-C/VEGFR-3 expression and lymph node metastases in squamous cell carcinomas of the larynx. The expression of these markers was positive only in the analysis of cervical metastases, and did not correlate with age, gender, T stage, or primary location of the laryngeal lesion.14
The antibody against epithelial growth factor receptor (EGFR) has already been approved by the FDA (Food and Drugs Administration) for a few years for the treatment of head and neck carcinoma. However, its use in patients with tumor recurrence and/or metastatic disease has shown little efficacy. VEGF has been implicated as a potential mechanism of resistance to anti-EGFR therapy. Therefore, recent clinical trials seek the use of anti-EGFR drugs in combination with anti-VEGF antibodies (bevacizumab), a combination that has been well tolerated by patients (Phase I/II studies).15–17
Currently, several phase II clinical trials have studied the role of bevacizumab in tumors of the head and neck, including its combination with cetuximab, cisplatin, and radiotherapy for non-metastatic stage III/IV cancer; with erlotinib in locoregional advanced and/or metastatic disease; and with cetuximab, docetaxel, and cisplatin (induction) followed by cetuximab, cisplatin, and radiotherapy in previously untreated locoregional advanced disease.18
The emergence of this promising treatment raises important issues. With broad indications, the indiscriminate use of bevacizumab can develop resistance. Therefore, studies have sought to define and validate biomarkers of its efficiency. Until then, specific VEGF genotypes and arterial hypertension (induced or aggravated by the treatment) appear to be related to a better response.19 Other markers such as CD105, CD34, and CD31, related to the process of angiogenesis, may be useful in the identification of patients who are candidates for more aggressive anti-neoplastic therapy, including the use of monoclonal antibodies.20
Some authors believe that the regulation of VEGF expression can be modulated by the microRNA-206, and this pathway can be used in the future for anticancer therapy related to this tumor marker.21
Although this study did not observe a correlation of VEGF with cervical lymph node metastasis, the targeted therapy has shown promising results for the treatment of head and neck cancer.22
ConclusionVEGF was scarcely expressed, and no correlation with cervical lymph metastasis spread was observed in this sample of 15 cases.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Bonhin RG, Rocha VB, de Carvalho GM, Guimaraes AC, Crespo AN, Chone CT, et al. Correlation between vascular endothelial growth factor expression and presence of lymph node metastasis in advanced squamous cell carcinoma of the larynx. Braz J Otorhinolaryngol. 2015;81:58–62.
Institution: Department of Otorhinolaryngology, Head and Neck, Universidade Estadual de Campinas (UNICAMP), Campinas, SP, Brazil.