To analyze the morphofunctional regeneration process of facial nerve injury in the presence of insulin-like growth factor-1 and mesenchymal stem cells.
MethodsFourteen Wistar rats suffered unilateral facial nerve crushing and were randomly divided into two groups. All received insulin-like growth factor-1 inoculation, but only half of the animals received an additional inoculation of mesenchymal stem cells. The animals were followed for 90 days and facial nerve regeneration was analyzed via spontaneous facial motor function tests and immunohistochemistry in the nerve motor nucleus.
ResultsThe group that received the growth factor and stem cells showed a statistically superior mean in vibrissae movements (p < 0.01), touch reflex (p = 0.05) and eye closure (p < 0.01), in addition to better immunohistochemistry reactivity. There was a statistically significant difference in the mean number of cells in the facial nerve nucleus between the experimental groups (p = 0.025), with the group that received the growth factor and stem cells showing the highest mean.
ConclusionThe association between growth factor and stem cells potentiates the morphofunctional regeneration of the facial nerve, occurring faster and more effectively.
Level of Evidence4, degree of recommendation C.
Facial paralysis refers to the interruption of motor information to the facial muscles, being responsible for functional and aesthetic disorders for the affected individuals. This is explained by the importance of the facial mimic muscle innervation in terms of eye protection, masticatory competence and transmission of emotions.1–5
In this context, the paralysis results from injuries to the facial nerve, which include different etiologies such as Bell’s palsy,6 damage resulting from surgical procedures such as parotidectomy,7 or inflammatory processes concomitant with infections.8
Considering the importance of understanding regeneration and the impact of new therapeutic strategies based on bioengineering, animal models are essential in the investigation process of neurological injuries.9–12 In this regard, the Insulin-like growth factor-1 (IGF-1) is a phylogenetically ancient neurotrophic hormone with endocrine, paracrine, and autocrine actions on the CNS maturation.13,14 Moreover, just as the origin in the facial nerve depends on the embryo’s neural crest differentiation,15 mesenchymal stem cells (MSCs) have been implicated in regenerative models.12,16
Considering the regenerative potential of MSCs and IGF-1 in peripheral nerve injuries, this study analyzed the application of both in facial nerve regeneration in Wistar rats after facial nerve crush injury, by quantitative and qualitative methods throughout the postoperative period.
MethodsExperimental designAll experimental procedures and protocols involving the animals were approved by the Animal Ethics Committee of Universidade do Estado do Rio Grande do Norte (UERN), under approval number 005–16, in accordance with the ethical principles adopted by the Brazilian Society of Science in Laboratory Animals (Sociedade Brasileira de Ciência em Animais de Laboratório). A total of 20 male Wistar rats, weighing approximately 300 grams were obtained from the institution’s animal facility. Mesenchymal stem cells were collected from six animals, and facial nerve injuries were performed in 14. These were randomly divided into groups and received facial nerve crush injury, with 7 animals submitted to facial nerve injury and inoculation of IGF-1, and 7 animals submitted to facial nerve injury and inoculation of MSCs + IGF-1.
Cell cultureThe MSCs were collected from the bone marrow of the femurs and tibias of two six-month-old Wistar rats, under aseptic conditions, by inserting a 26-gauge syringe into the bone cavity, washing it with 10 mL of KnockOut Dulbecco’s Modified Eagle Medium (DMEM) low glucose.
The newly dissected bones were kept in a conical tube with phosphate-buffered saline (PBS, pH 7.4) until all muscle tissue adhered to the bones was removed. Under laminar flow and in a sterile medium, the epiphyses of the bones were cut, exposing the bone marrow. Using a 1-mL syringe, the marrow was extracted through insertion into the spinal canal and subsequent washing of the canal using 1 mL of culture medium under pressure. The cells were seeded in 60 mm petri dishes for cell culture with 5 mL of DMEM medium supplemented with 10% of Fetal Bovine Serum (FBS) and kept in an incubator with 5% CO2 at 37 °C.
An inverted optical microscope with phase contrast was used to observe cell adhesion at the bottom of the plates. To allow an adequate nutritional supply, eliminate hematopoietic and detached cells and adhere the cells to the bottom of the plates, the culture medium was changed every three days. The culture medium used was the KnockOut DMEM low glucose, supplemented with 10% fetal bovine serum and 10 U/mL of penicillin G, 10 ug/mL of streptomycin and 25 ug/mL of amphotericin B, all obtained from GIBCO Invitrogen Corporation®.
In the first subculture, upon reaching 70%–90% of confluence, the basic medium was removed using a suction pump and 2 mL of trypsin-ethylenediaminetetraacetic acid (EDTA) were added (0.25% trypsin containing 1 mM EDTA – Cutilab/Brazil). The cell suspension was placed in a conical Falcon tube with the same volume of Alpha Minimum Essential Medium (MEM) supplemented with 20% PBS, in order to inactivate trypsin. The suspension was centrifuged at 1200 rpm for ten minutes, after which the supernatant was discarded and the cells were resuspended in 1 mL
Aliquots were obtained for counting the number of cells in a Neubauer chamber. They were deposited on 18 P60 plates and cultivated for three periods of time: 24, 48 and 72 h, with 6 plates for each time interval. Using this procedure, it was possible to evaluate the adhesion and proliferation of MSCs, establishing a cell growth curve in both groups. After the study time intervals, the cells were trypsinized and the number of cells and cell viability were analyzed: the aliquot withdrawn for cell counting was added to the same volume of Trypan Blue, and then viable and non-viable cells were counted.
The number of cells collected from each plate was obtained by counting viable cells using a hemocytometer and the trypan blue staining method of exclusion for non-viable cells, following Freshney’s recommendations.17 To calculate the number of cells, the following formula was used: NC × D/ # Q (NC = Number of vital Cells counted, D = sample Dilution and #Q = Number of Squares of the hemocytometer used for counting the cells). The percentage of viability of the cell population was obtained by dividing the total number of viable cells by the total number of cells and the result was multiplied by 100.
Inoculation of IGF-1 in the presence of stem cells (SCs) in the facial nerveIGF-1 inoculation was carried out with a 25 gauge Hamilton syringe at a rate of 10 mL/min. The syringe was held in place for one minute to allow cell dispersion to occur. The MSCs supplemented with IGF-1 were inoculated directly into the nerve, facilitating the absorption.
Surgical procedure and euthanasiaThe animals were anesthetized with 10% ketamine hydrochloride (0.1 mL/100 g) and 2% xylazine (0.01 mL/100 g) intraperitoneally and with inhaled isoflurane until the end of the surgery. Then, they were submitted to right retroauricular trichotomy; a vertical retroauricular incision was made, involving the skin, subcutaneous tissue and platysma muscle; blunt dissection and identification of the tendinous margin of the clavotrapezius muscle, the trunk and bifurcation of the right facial nerve and the external jugular vein. The crushing of the facial nerve was performed with a number 14 Kelly forceps, in the extratemporal segment, before the bifurcation; the skin and subcutaneous tissue were sutured using separate stitches with nylon 4.0 thread.
After the surgery, the animals were followed for 90 days on a shelf with adequate temperature and bacteriological flow, with a 12 -h light/12 -h dark cycle. The rats received antibiotic, ceftriaxone 40 mg/kg/day, intramuscularly in the hind limbs for 10 days. They were housed in cages and had free access to water and food. At the end of this period, the animals were euthanized with an excessive dose of anesthetics (xylazine and ketamine from Agener União®) intraperitoneally, where the dose was calculated according to the animal’s weight, requiring the application of three-fold the anesthetic dose of the association of ketamine and xylazine hydrochloride. Facial nerve segments adjacent to the injury were removed and fixed in the fixative solution for 90 min and washed in 10% sucrose solution dissolved in phosphate buffer for 48 h.
Behavior of the facial motor functionThe animals were observed for spontaneous facial motor function (vibrissae movement, eye opening diameter, vibrissae reflex to touch, upper eyelid resistance to lifting and observation of eye closure) comparing the injured to the non-injured side during the rats’ typical movements when exploring the environment.18
A value was assigned according to a numerical scale to assess the reflex of the vibrissae to touch (1 ‒ No response to touch; 2 ‒ Mild response; 3 ‒ Moderate response; 4 ‒ Normal response), and a scale was also applied to the observation of the movement of the vibrissae (0 ‒ No movement; 1 ‒ Mild tremor; 2 ‒ Effective movement with posterior positioning and amplitude and frequencies lower than the contralateral ones; 3 ‒ Effective movement with positioning similar to the contralateral one, but with inferior amplitude and frequencies; 4 ‒ Effective movement with positioning and amplitudes similar to the contralateral ones, but with a lower frequency; 5 ‒ Effective movement with positioning, amplitude and frequencies similar to the contralateral ones).19
The animals' eye closure was observed through an air jet stimulus, produced by the rapid compression of the plunger of a 20 mL syringe directed against each eyeball. Here, the injured side was not compared to the non-injured side, since the lateralized positioning of the eyeballs does not allow simultaneous bilateral stimulation.
The quantification of the eye opening was as follows: 1 ‒ 1-mm eye opening; 2 ‒ 2-mm eye opening; 3 ‒ 3-mm eye opening; 4 ‒ 4-mm eye opening; 5 ‒ 5-mm eye opening. Taking into account the ocular rim closure, a numerical value was assigned according to a numerical scale (0 ‒ No perceptible movement; 1 ‒ Contraction, but no perceptible ocular rim closure; 2 ‒ Closure of up to 25% of the ocular rim; 3 – Closure of 25%–50% of the ocular rim; 4 – Closure of 50%–75% of the ocular rim; 5 – Closure of 75%–100% of the ocular rim). Resistance to the lifting of the upper eyelid was evaluated for the existence of: 1 ‒ Absent resistance; 2 ‒ Light resistance; 3 ‒ Moderate resistance.19
It is worth mentioning that the analysis of facial motor function behavior was performed by an investigator blinded to the study group.
In addition to the functional assessments of the facial nerve, a study of the motor nucleus of the facial nerve in the brainstem was carried out through an immunohistochemical study, to evaluate the regeneration and influence of the inoculation of IGF-1 and mesenchymal cells at the central level.
Material sectioningThe nerve segments were reduced to 1 cm and frozen with dry ice (−45 °C). The sections were mounted on gelatinized slides and processed for immunohistochemistry. A last serial section was stored in 30% sucrose buffer solution to obtain scanning electron microscopy images. Subsequently, the animals were decapitated and their heads dipped in 70% ethanol.
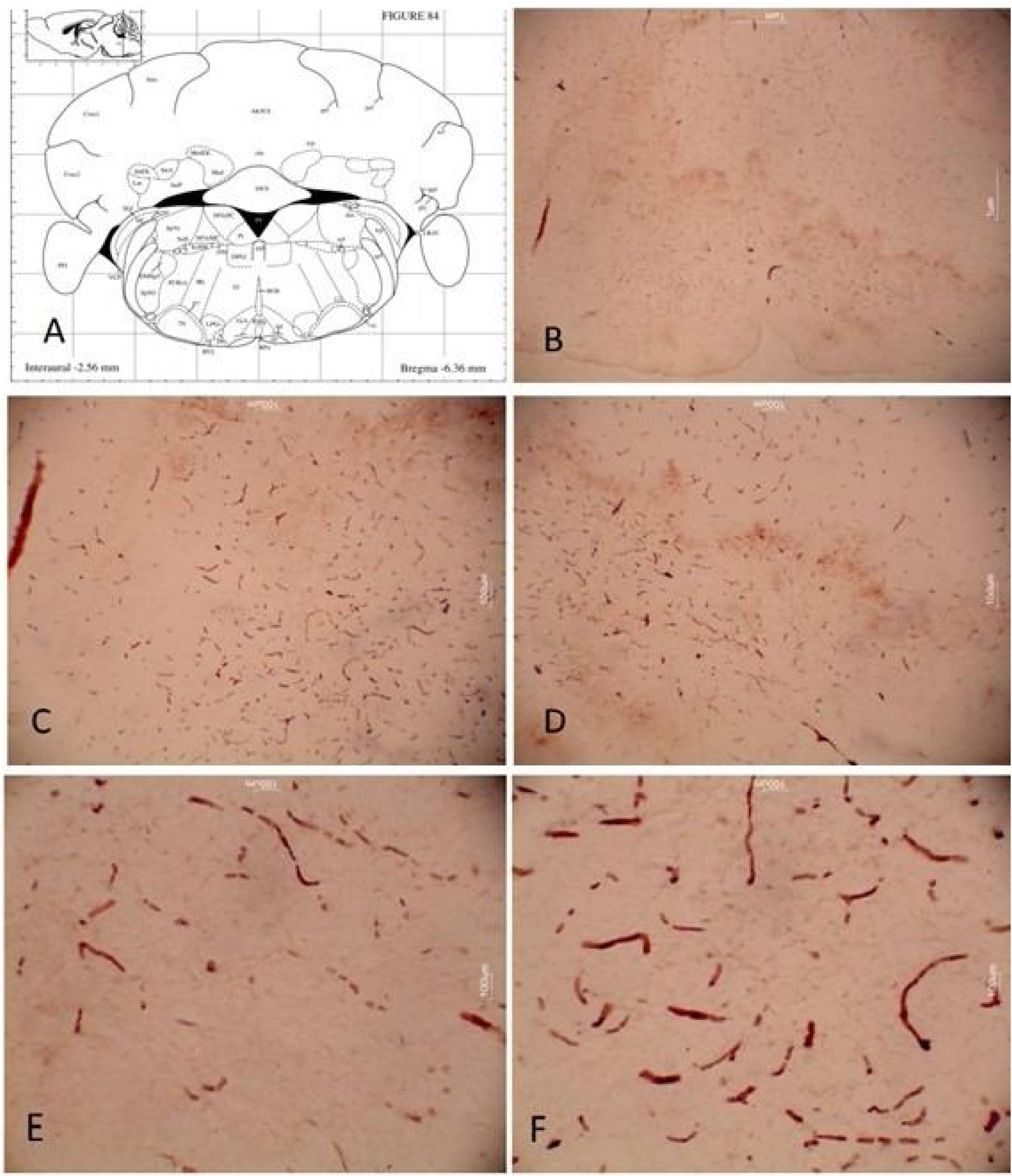
The brains were removed and placed in a 100-mm diameter glass plate (Corning, USA) containing 10 mL of an ice-cold, pH 7.4 sterile solution, consisting of: 120 mM NaCl, 5 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO3, 10 mM Hepes, and 13 mM glucose. The meninges and blood vessels were removed using specific microsurgical instruments (Roboz Surgica USA) under a dissecting microscope (DF Vasconcelos, Brazil). The brainstem was identified using the same criteria used by Kivell et al.20 Then, the brainstem of the animals was placed on 100 mm glass plates containing the same sterile solution described before. The region corresponding to the facial nerve emergence at the level of the pons was identified and submitted to embedment for sectioning.9,20
Immunohistochemical stainingThe microdensitometric immunohistochemical method was performed according to Humpel et al.21 With a random start, sections from the studied regions were systematically sampled. Slides with facial nerve sections related to the epicenter and facial nucleus were immersed in a petri dish soaked in 0.1 M phosphate buffer, where the slides remained for 5 min. The sections were washed 3 times for 5 min in 0.1 M phosphate buffer plus 0.5% Triton X-100.
The sections were maintained in 10% fetal bovine serum diluted in phosphate buffer plus 0.5% triton X-100 for 30 min. The tissues were incubated for 24 h at 20 °C with one of the immunohistochemical markers according to the protocol: brainstem (facial nerve nucleus), growth-associated protein-43 (GAP-43) made in mice (Abcam) for identification of the axonal budding at a concentration of 1:2600.
The indirect immunoperoxidase system employing the avidin-biotin (ABC) system (Vectastain, USA) was used with 3-3’-diaminobenzidine tetrahydrochloride (DAB, Sigma) as the chromogen. After washed in phosphate buffer, the sections were incubated with biotinylated immunoglobulins (Vector, USA, diluted at 1:200) for one hour. These immunoglobulins were obtained from sheep and produced against the proteins of animals from which the primary antibodies were obtained. The antibodies were diluted in phosphate buffer containing 0.5% Triton X-100. Avidin and a biotinylated peroxidase were introduced (Vectastain, Vector, USA; both diluted at 1:100) in a third incubation for 45 min.
The reaction was completed with 0.03% DAB (Sigma) and H2O2 (Sigma) for 10 min. Immunohistochemical procedures were standardized. Thus, the DAB saturation point, the dilution of the primary antibody far from saturation and an adjusted incubation time so that the darker elements of the sections were below the saturation were considered. After the end of the immunohistochemistry battery, the enamel was removed from the slides and dried for one hour. Then, tissue dehydration was performed to demonstrate immunoreactivity. Entellan was added over the sections and glued to the coverslip.
Stereological quantitative analysisThe series of sections immunostained for GAP-43 for identification of the axonal budding at a concentration of 1:2600 were examined under a bright field microscope (Olympus BX41). Counts and measurements were performed using ImageJ software. The results were documented in photomicrographs and graphs.
Statistical analysisThe research database was built using the SPSS software platform. Initially, a descriptive analysis of all data was performed. Aiming to determine the levels of significance of the effects of the performed treatments, the data were submitted to the two-way analysis of variance (ANOVA) with repeated measures and ANOVA with Turkey and Bonferroni tests. Student’s t-test was used for independent measures and Levene’s test to verify equal variance between groups. A value of p < 0.05 was accepted as a statistically significant result.
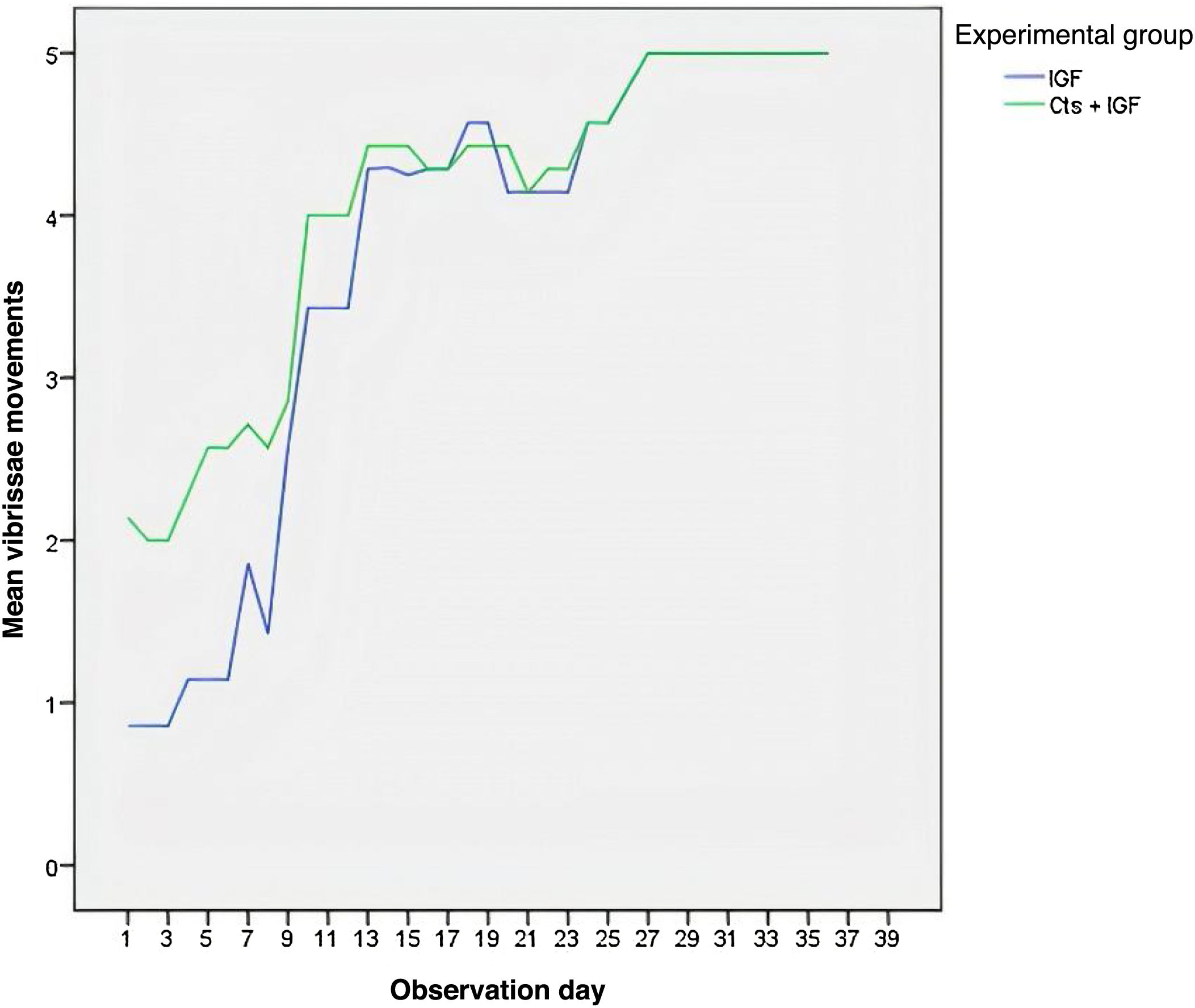
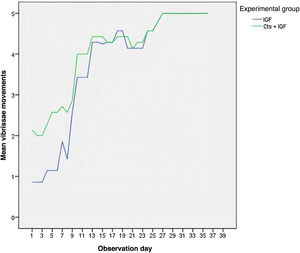
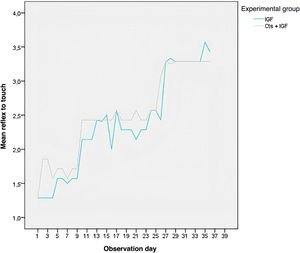
ResultsBehavioral resultsAs shown in Table 1, the MSCs + IGF-1 group showed a statistically higher mean than the IGF-1 group in the analysis of vibrissae movements, touch reflex and eye closure. The IGF-1 and MSCs + IGF-1 groups showed greater difference in vibrissae movements in the first days of observation, noting that the MSCs + IGF-1 group showed more movement (Fig. 1). This difference decreased with the progression of days as the movements increased.
Comparison of vibrissae movements, eye opening, touch reflex, eye closure and eyelid lifting between groups inoculated only with IGF-1 and inoculated with IGF-1+MSCs.
| n | Mean | Standard deviation | p-value | |
|---|---|---|---|---|
| Vibrissae movements | ||||
| IGF | 279 | 3.77 | 1.61 | <0.01 |
| SCs + IGF | 281 | 4.09 | 1.25 | |
| Eye openinga | ||||
| IGF | 279 | 2.58 | 1.03 | 0.59 |
| SCs + IGF | 281 | 2.52 | 1.04 | |
| Touch reflex | ||||
| IGF | 279 | 2.38 | 0.84 | 0.05 |
| SCs + IGF | 281 | 4.10 | 0.77 | |
| Eye closurea | ||||
| IGF | 279 | 3.82 | 1.39 | <0.01 |
| SCs + IGF | 281 | 4.10 | 1.11 | |
| Eyelid liftinga | ||||
| IGF | 279 | 2.81 | 0.98 | 0.24 |
| SCs + IGF | 281 | 2.90 | 0.90 |
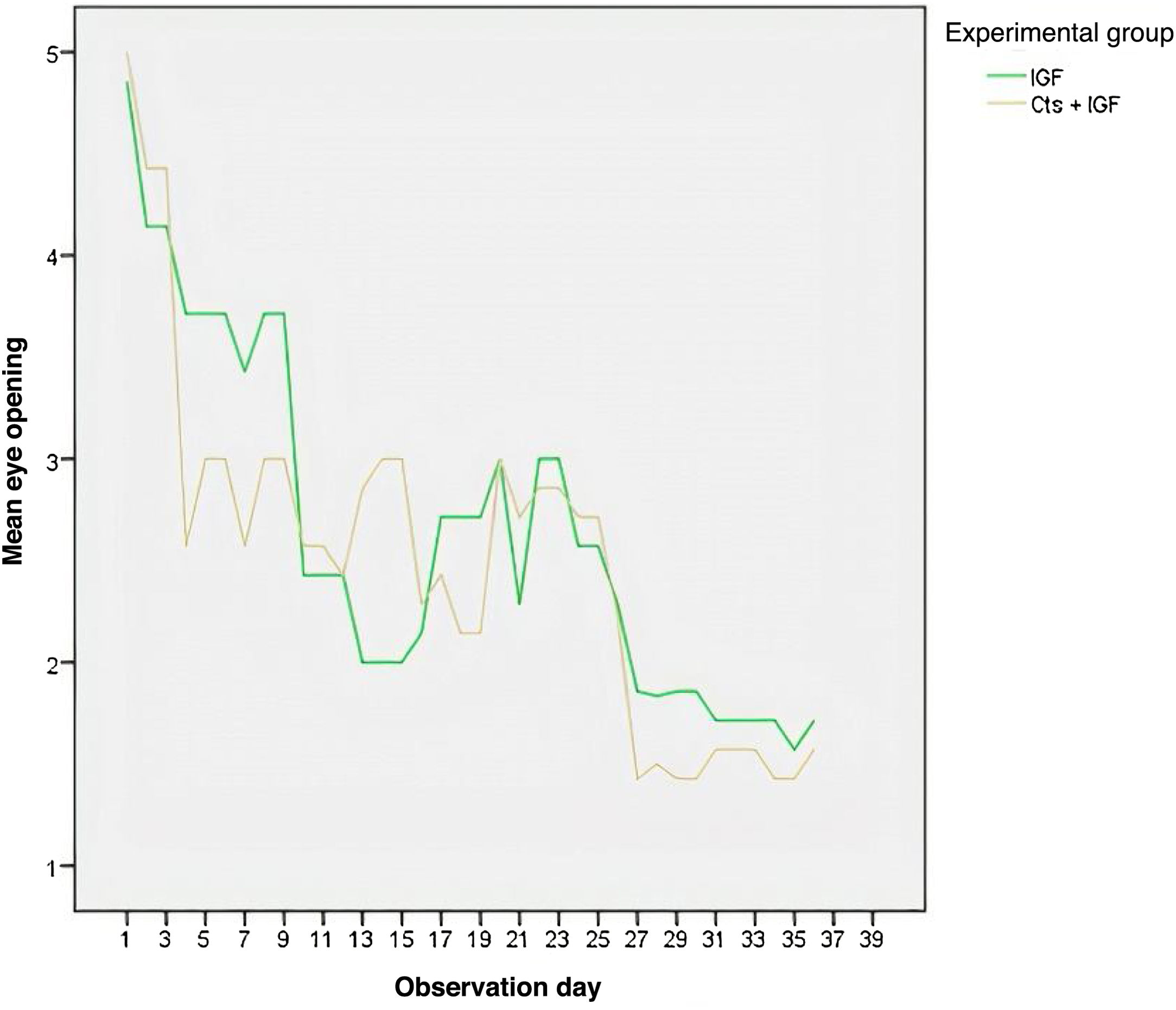
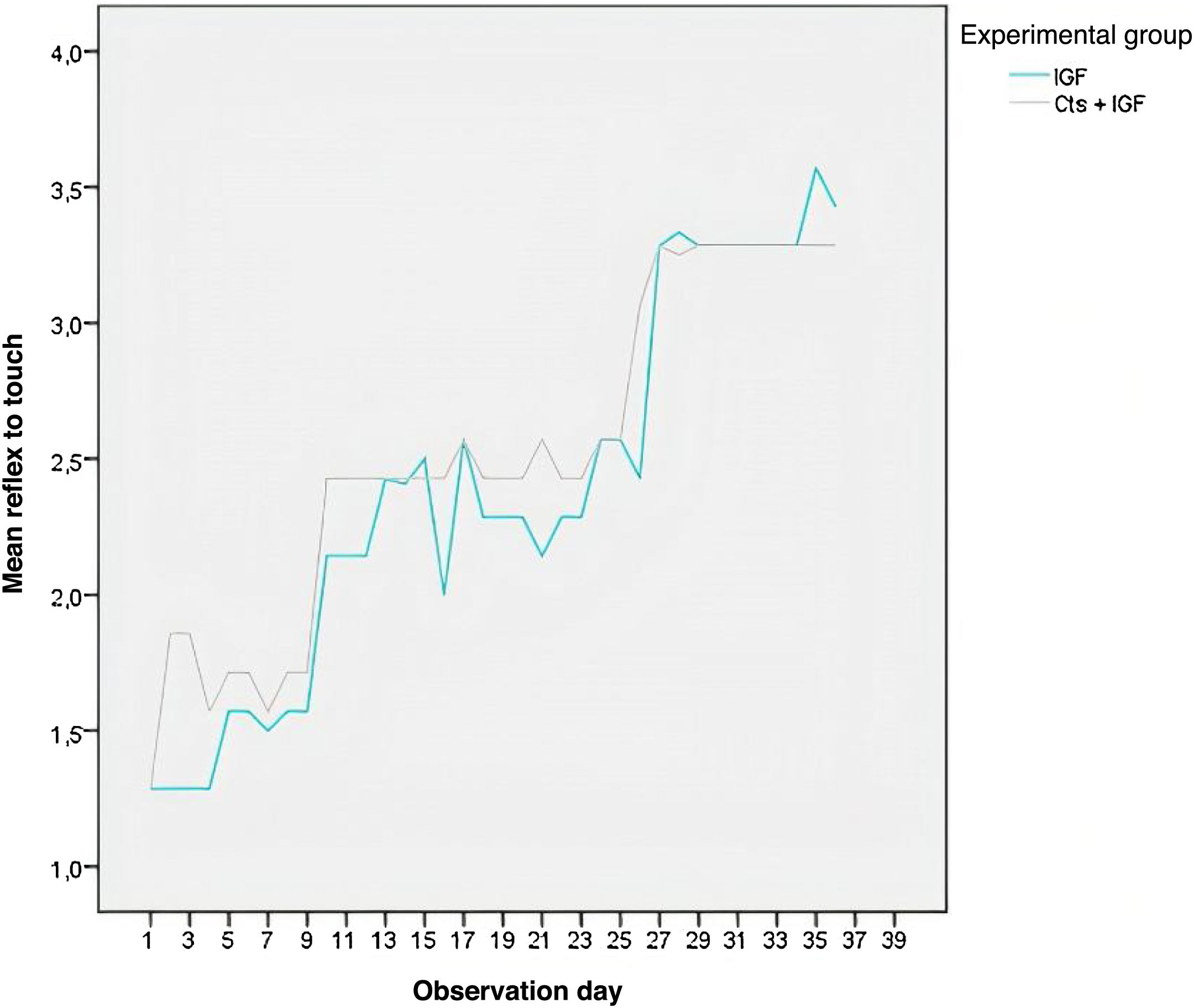
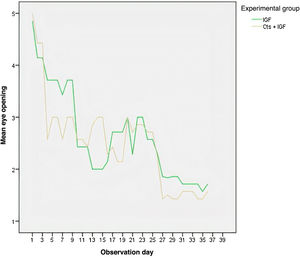
As shown in Fig. 2, the eye opening decreased in the two experimental groups with the days of observation. On most days, the MSCs + IGF-1 group had the lowest mean eye opening, except between days 11 and 15, when the group had a higher mean. Fig. 3 shows that the MSCs + IGF-1 group was, on most observation days, superior to the IGF-1 group in terms of the touch reflex.
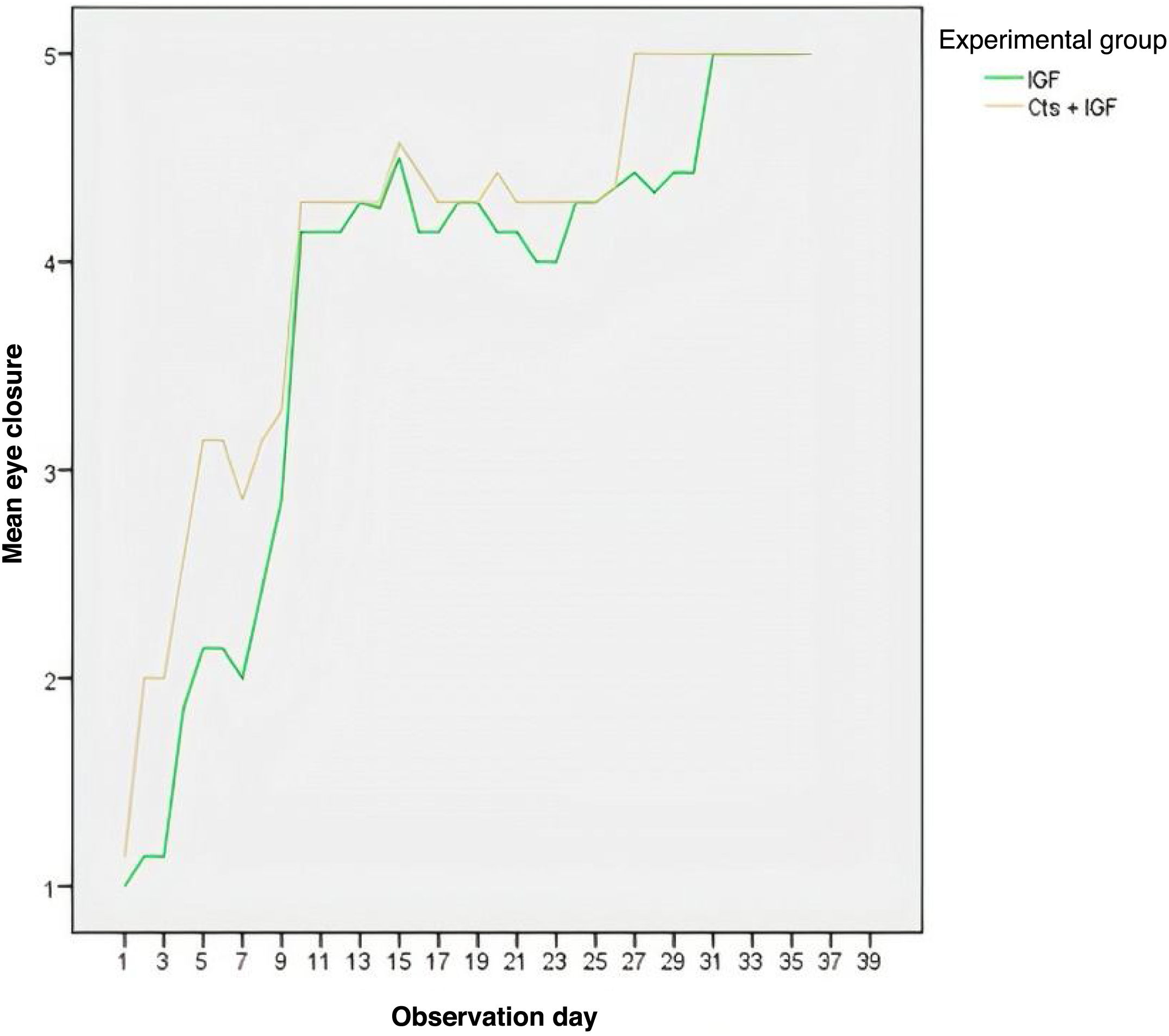
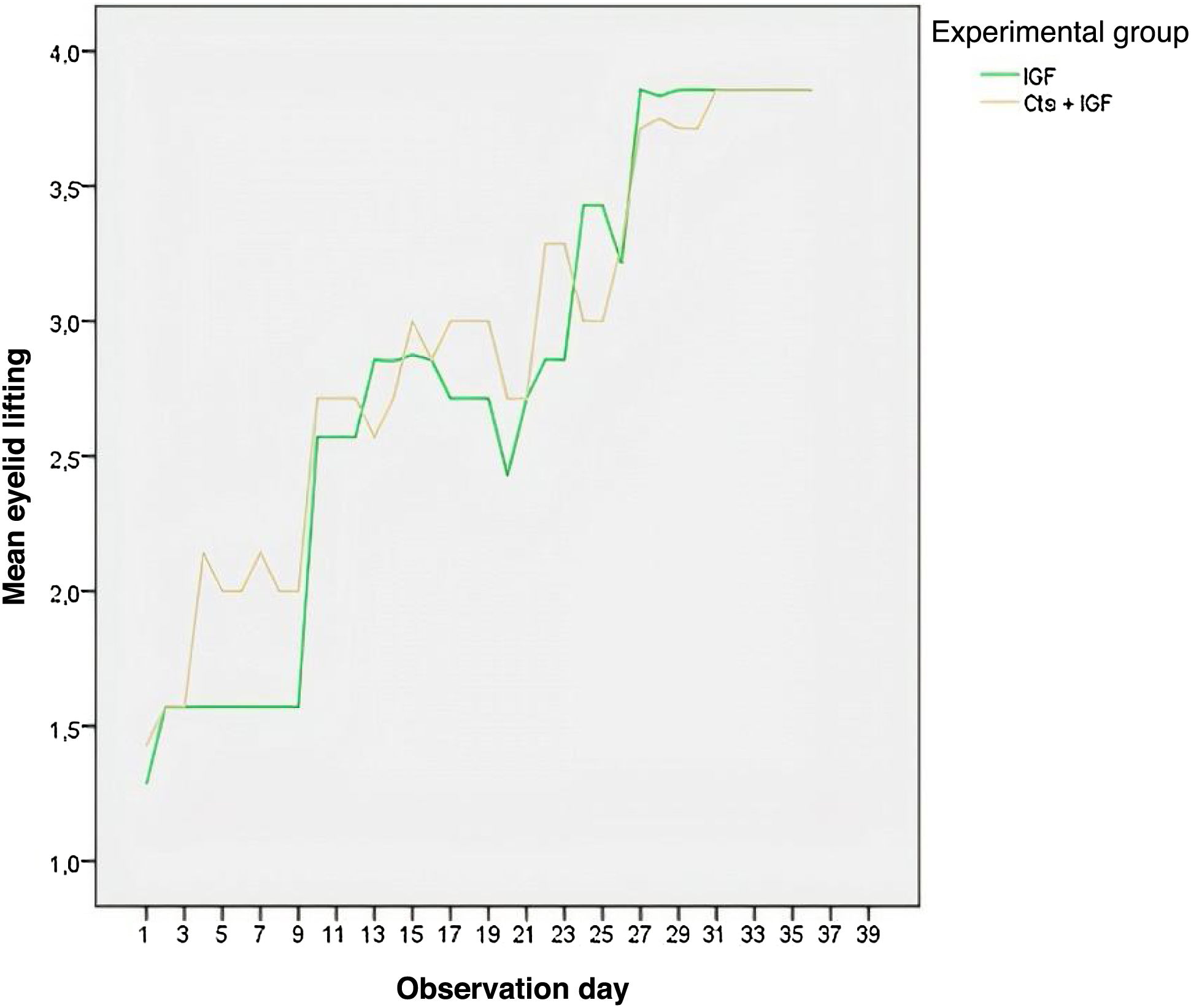
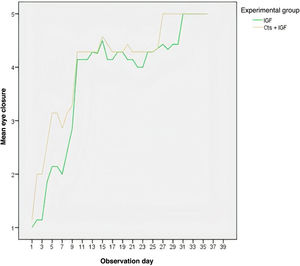
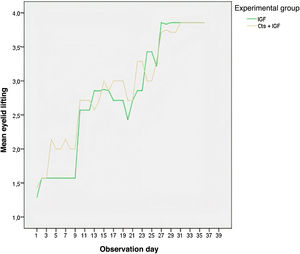
According to Fig. 4, the MSCs + IGF-1 group showed a higher mean of eye closure for all observation days, except for the last three days, where the curves representing eye closure overlapped. The group that received the inoculation of MSCs + IGF-1 also had a higher mean of eye closure for almost all observation days (Fig. 5).
The cell number comparisons between the IGF-1 and MSCs + IGF-1 groups were performed with 36 Student’s t-tests and bar graphs. A p-value < 0.05 was accepted as statistically significant. Although the MSCs + IGF-1 group had a higher mean number of cells (M = 22.00), this difference was not within what is considered statistically significant for this study. There was a statistically significant difference in the mean number of cells between the experimental groups (p = 0.025), with the MSCs + IGF-1 group exhibiting a higher mean when compared to the IGF-1 group.
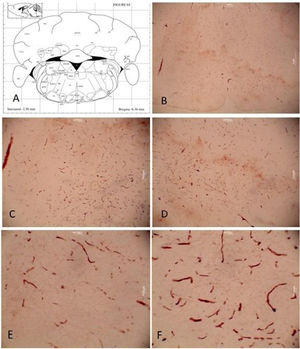
Fig. 6 shows the representation of the facial nerve nucleus. For the IGF-1 group, the operated right side had a higher mean number of cells; however, there was no statistically significant difference between the two sides. On both sides, the MSCs + IGF-1 group had a higher number of cells compared to the IGF-1 group. In the experimental groups, the right side showed a greater number of cells compared to the left side.
DiscussionIGF-1 has been identified as responsible for significant effects on adaptive changes made by the CNS in the presence of the replacement of functional demands, acting on cell neuroplasticity.11,22–27
It has been suggested that the presence of mesenchymal stem cells at the site of crush injuries in Wistar rats may act as a paracrine communication triggered by secreted trophic factors and cytokines, such as IGF-1, which act as facilitators of the migration, proliferation and activation of SCs by promoting the endogenous repair of a damaged area, resulting in functional recovery.28–31
Several studies have shown that growth factors can induce neural differentiation of mesenchymal stem cells. As in the study by Kang et., 2013, when observing the neural differentiation of muscle-derived stem cells (MDSCs) after treatment with basic fibroblast growth factor (BFGF) and ethosuximide, the cells were isolated from the rat skeletal muscle (rMDSCs) and pre-induced by culture with 25 ng/mL of BFGF for 24 h and then transferred to a medium supplemented with or without Methosuximide. In the study by Shi et al. (2009), functional (electroneuromyography) and histological (qualitative and quantitative) evaluations were performed to reach the conclusion that the combination of glial-derived neurotrophic factor with neural stem cell transplantation can improve regeneration in the peripheral nervous system.
In the present study, specimens with crush injuries were randomly divided into two groups, one of which was inoculated with IGF-1 only, while the other was inoculated with IGF-1 associated with MSCs. It is known that when a nerve fiber is crushed, the part of the axon distal to the injury undergoes Wallerian degeneration for 1–2 weeks, a period during which IGF-1 is present and activates the signal transduction system in the cell. This analysis demonstrated that the group that received an association of MSCs + IGF-1 had a statistically superior mean, when compared to the group that used only IGF-1, for the following variables: vibrissae movements (p < 0.01), touch reflex (p = 0.05 ) and eye closure (p < 0.01) on the first nine days of observation.
These results corroborate what was demonstrated in the immunoreactivity for GAP-43, so that the group that associated IGF-1 with MSCs also showed better results. There was a statistically significant difference in the mean number of cells in the facial nerve nucleus between the experimental groups (p = 0.025), with the group that received the growth factor and stem cells exhibiting a higher mean, which can be explained by better nerve plasticity in the group that associated IGF-1 and MSCs. Moreover, MSCs lack HLA-DR expression (main histocompatibility complex class II antigen), which makes them significantly less immunogenic than other cell types.
Although the facial nerve originates in the CNS, it has greater ability to regenerate when compared to CNS neurons, because it is a peripheral nerve sheathed in Schwann cells (SC), although it shares a central glial environment.
One limitation is that a distal portion of the extratemporal facial nerve was injured, because it is easy to expose the nerve running subcutaneously outside the temporal bone.32–36 However, this model does not accurately reflect the situation experienced by patients with peripheral facial nerve paralysis.32 Other limitations are the use of bone marrow-derived MSCs, as dental pulp stem cells have clinical advantages due to their simplicity, low extraction morbidity, higher in vitro proliferation rates, and high efficiency in forming adherent colonies.37–41
ConclusionThe extratemporal application of IGF-1 and MSCs resulted in an improvement in the degree of eyelid closure, touch reflex and vibrissae movement throughout the total study period. There was a significantly higher mean number of cells in the facial nerve nucleus in a group treated with IGF-1+MSCs. Additional experiments should be conducted prior to clinical trials for the treatment of facial nerve paralysis in humans.
Conflicts of interestThe authors declare no conflicts of interest.