Eosinophilic otitis media is an intractable otitis media and a fairly common middle ear disease. However, the pathogenesis of eosinophilic otitis media is obscure.
ObjectiveTo observe the pathological and ultrastructural changes of the Eustachian tube mucosal epithelium in rats with eosinophilic otitis media and further explore the pathogenesis of eosinophilic otitis media.
MethodsAnimals were intraperitoneally injected with 2000 mg ovalbumin and 100 mg aluminum hydroxide (alum) on day 0, followed by 100 mg ovalbumin and 100 mg alum injection on days 7 and 14. Next they were topically boosted by daily application of 100 mg ovalbumin solution via nasal drip and intratympanic injection of 0.1 mL ovalbumin (1000 mg/mL) in the right ear (group A, n = 80) and 0.1 mL saline in the left ear as control (group B, n = 80) starting on day 21 and continuing for 14 days. The temporal bones were dissected on the 35th, 38th, 41st and 43rd day separately under anesthesia. Scanning electron microscopy, hematoxylin-eosin and toluidine blue staining were used to observe the pathological and morphological changes of Eustachian tube mucosa stained samples. Moreover, inflammatory cells and cilia were counted.
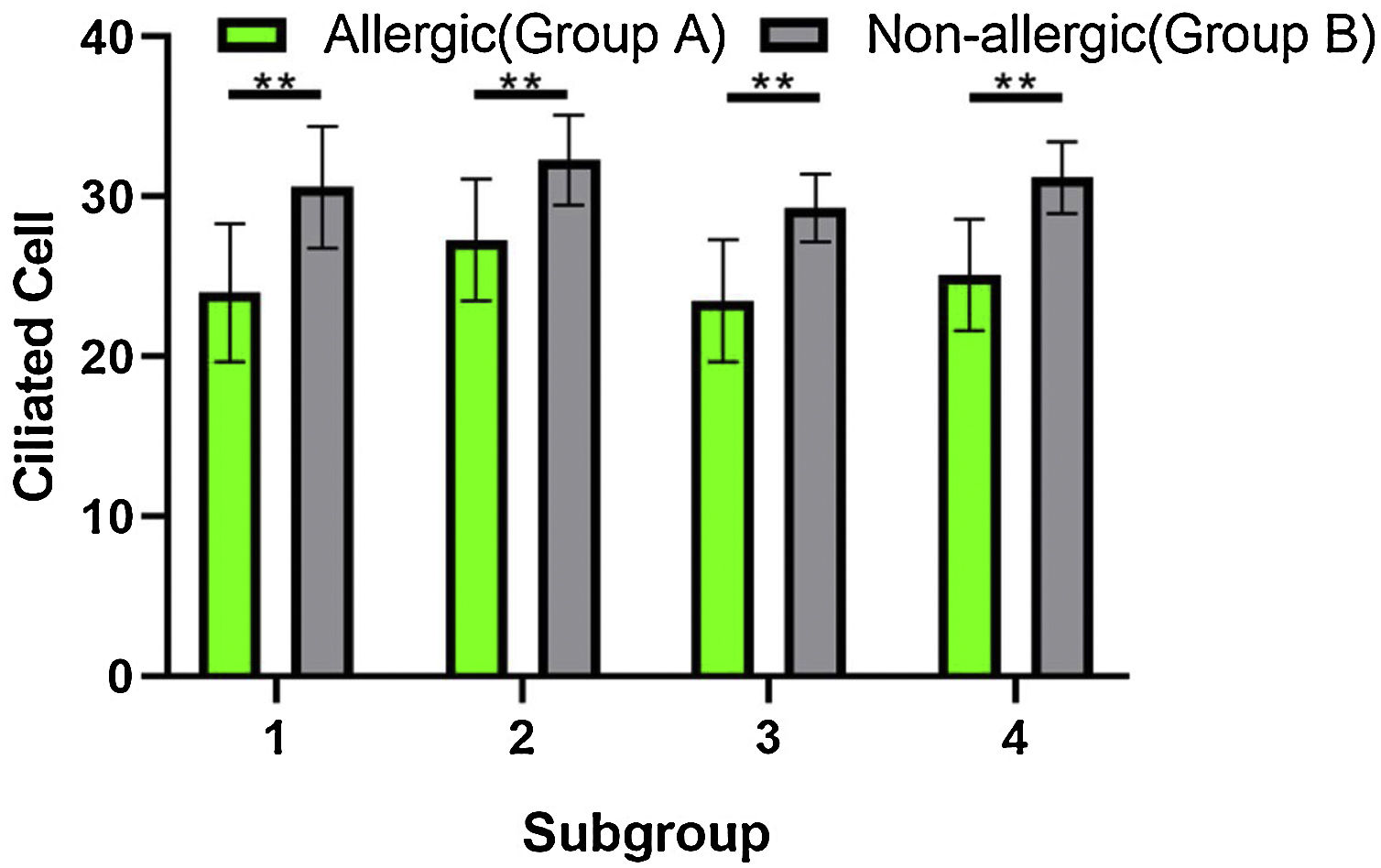
ResultsThe epithelium of the Eustachian tube in group A was swollen and thickened. The cilia were arranged in a disorderly manner and partially detached. Eosinophils infiltrated the submucosal layer of the Eustachian tube, and their number increased significantly compared with that in group B (p < 0.05). Simultaneously, mast cell degranulation was observed in group A. Scanning electron microscopy revealed that the cilia were lodged and gathered along the whole length of Eustachian tube in group A. Ciliated cell density was significantly lower than that in Group B (p < 0.01).
ConclusionIn the eosinophilic otitis media model, allergy caused significant changes in pathology and morphology of the Eustachian tube mucosa, affecting the normal function of the Eustachian tube which played an important role in the occurrence and development of eosinophilic otitis media.
Otitis media with effusion (OME) predisposes preschool children to conductive hearing loss. A previous study found that about 90% of preschool children and 12.5% of school-age children have suffered from OME, especially between the ages of 6 months to 4 years, which seriously affects their language formation and development due to the long duration of treatment.1,2 Recently, the clinical incidence of OME has increased; however, its pathophysiology has not yet been clarified. According to the concept of inflammation of the upper and lower respiratory tract, the middle ear mucosa system is the continuation of the upper respiratory tract as a potential immune organ.3–5 Epidemiological studies have found that OME is more likely to be a comprehensive manifestation of atopic allergic diseases such as allergic rhinitis, eczema, and asthma.6–12 Accumulating clinical observations suggest that some patients with OME do not exhibit acute inflammation symptoms, while others present the symptoms even after the acute phase has passed.13–19 There have been many reports on patients with intractable OME or chronic otitis media associated with bronchial asthma and/or allergic rhinitis. Tomioka et al.20 named the specific-type intractable otitis media associated with bronchial asthma as eosinophilic otitis media (EOM) because skin testing did not detect type I allergy to a specific allergen in some patients despite the presence of eosinophils. Eosinophils are effector cells in the pathogenesis of allergic diseases and play an important role in the occurrence of EOM. Iino et al. proposed the diagnostic criteria for EOM based on the analysis of clinical characteristics in 138 patients diagnosed with EOM in five centers participating in the EOM study group (Table 1).20
Diagnostic criteria of EOM.
| Major criterion | Minor criterion |
|---|---|
| Otitis media with effusion or chronic otitis media with eosinophil-dominant effusion | 1. Highly viscous middle ear effusion |
| 2. Resistance to conventional treatment for otitis media | |
| 3. Association with bronchial asthma | |
| 4. Association with nasal polyposis | |
| Definitive case: positive for major + two or more minor criteria | |
| Exclusion criteria: Churg-Strauss syndrome, hypereosinophilic syndrome | |
In middle ear effusion, eosinophil chemoattractants such as IL-5 and eotaxin were found and the expression of eosinophil chemoattractants such as eotaxin (regulated on activation, normal T expressed and secreted) and ecalectin mRNAs were also found in the middle ear mucosa by in situ hybridization. These findings indicate that active eosinophilic inflammation occurs locally in the middle ear.21–23 Anti-IL-5 therapy using mepolizumab was effective at inhibiting eosinophilic recruitment to the middle ear in patients with EOM.24,25
Currently, EOM is a fairly common and intractable middle ear disease. Although studies have revealed the pathological conditions of the middle ear in EOM and the presence of numerous activated eosinophils and the eosinophil cationic protein (ECP) in the thickened middle ear mucosa, the presentation in the ET is obscure. This study aimed to determine the relationship between EOM and ET through experiments on the EOM rat model using scanning electron microscopy (SEM) and HE staining to observe the pathological and ultrastructural changes of the ET mucosal epithelium in rats, to further explore the pathogenesis of EOM and to provide a new rationale for this phenomenon.
MethodsExperimental materialsFor power calculations, an equal standard deviation in groups A and B was assumed. Eighty healthy male Sprague-Dawley (SD) rats (age 6–8 weeks, weight 250–300 g). The animal experiments were approved by the Institutional Animal Care and Use Committee (Permit Number: AEEI-2018-102). All efforts were made to minimize the number of animals used and their suffering.
Establishment of an SD rat model of OMEFor the modeling of OME in group A, Nishizawa H’s modeling methods were consulted.26 Briefly, 80 rats were intraperitoneally injected on day 0 with 2000 mg Ovalbumin (OVA) and 100 mg aluminum hydroxide (alum) and on days 7 and 14, with 100 mg OVA and 100 mg alum for general sensitization. Then, starting on day 21 and for the next 14 days, the animals were topically boosted by daily application of 100 mg OVA solution by nasal drip and intratympanic injection of 0.1 mL OVA (1000 mg/mL) in the right ear (group A), and 0.1 mL saline in the left ear for control (group B). All procedures were carried out under anesthesia with 10% chloral hydrate (0.1 mg/kg, i.p.).
Sample of rat ETAfter the final OVA injection, the animals were deeply anesthetized with 10% chloral hydrate. The temporal bones from the right (group A) and left (group B) ears were dissected on the 35th, 38th, 41th and 43thday, resulting in eight subgroups: group A1, A2, A3, A4 (right ear, n = 20 per subgroup) and group B1, B2, B3, B4 (left ear, n = 20 per subgroup). Next, the samples of bilateral ETs were completely taken under magnification with the operating microscope (Leica, Germany), retaining the integrity of the ET. Bilateral ETs were cut along the long axis into upper and lower parts. One part was used to observe the surface of the mucosal tissue under SEM and the other was used for observation with HE and toluidine blue staining.
Preparation and observation of tissueHE staining and paraffin sections of the surface mucosal tissue on one ET part from each group were performed and then observed under an optical microscope (Nikon, Japan). First, morphological changes in the mucosal epithelium, including mucosal thickening, cilia epithelial swelling, cilia alignment, and cilia shedding, were observed. Second, eosinophils and neutrophils from three randomly selected views were counted under ×1000 magnification. Mast cells were counted using the same method after staining with toluidine blue. The mean values were recorded as data.
The surface mucosal tissue of the other ET part from each group was observed under SEM (Leica, Germany) after embedment and gilt. Pathological changes in the ultrastructure of the mucosal epithelium, including cilia loss, lodging, and gathering, were observed at different magnifications. The number of ciliated cells was counted in five areas of approximately 80 × 40 μm; one was selected 60 μm away from the tympanic cavity at the lowest point of the concave surface of ET and four were selected from the surface of both sides. The mean values were taken as data.
Statistical analysisAll animals used in this study were males and were randomly divided into eight subgroups. The data were statistically analyzed using SPSS 17.0 statistical software. Results are expressed as mean ± standard deviation. If no special labeling is used, then the t-test was used for statistical analysis. Differences were considered statistically significant at p < 0.05.
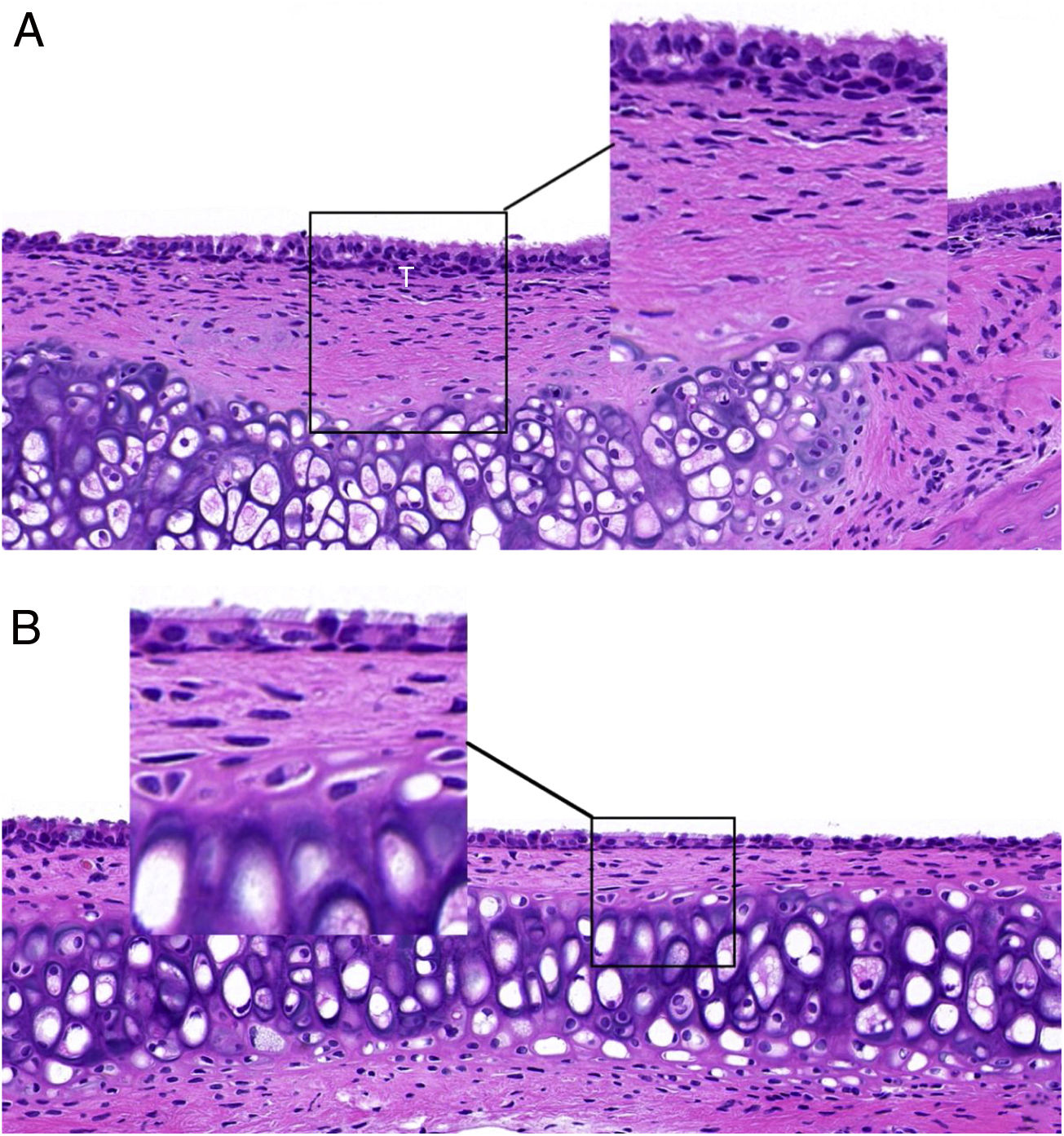
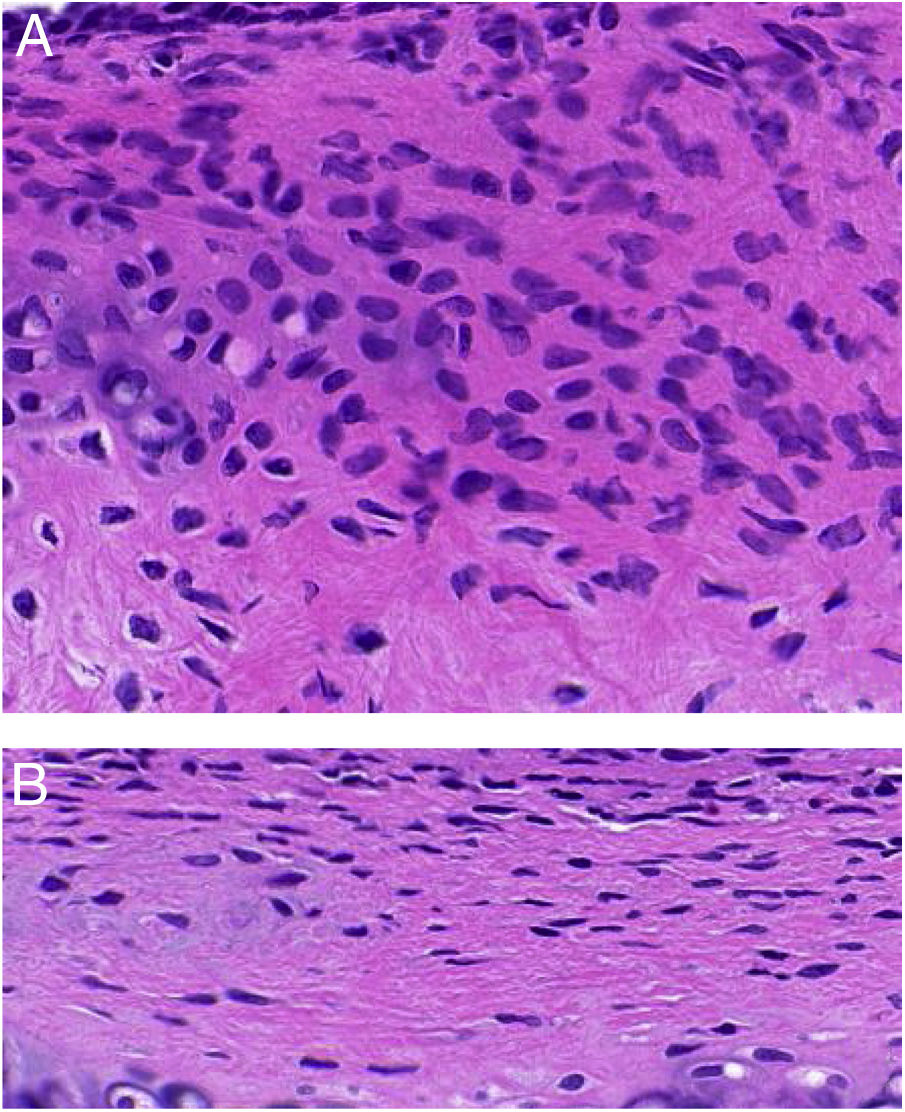
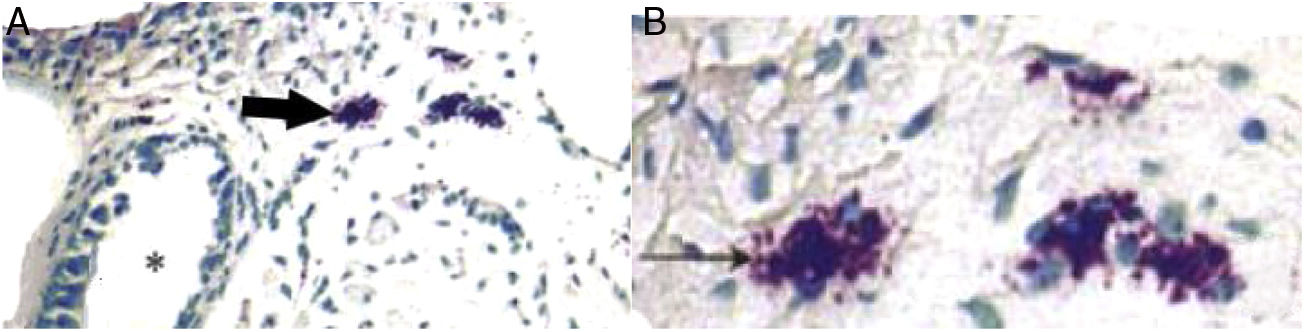
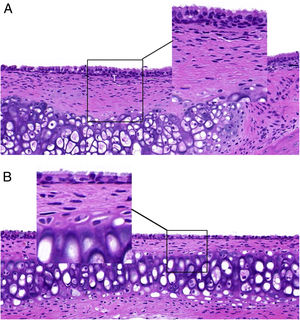
ResultsET mucosal pathologyHE is staining of the ET mucosa under an optical microscope revealed that the ciliated epithelium was slightly swollen, and the cilia structure was normal and neatly arranged in the control group (group B) (Fig. 1B). By comparison, in the EOM group (group A) there was obvious ciliated epithelial cell swelling on the ET, disordered and shedding cilia, the ET mucosa was thickened, and capillaries were enlarged and expanded (Fig. 1A). The numbers of eosinophils and neutrophils increased compared with those from group B (Fig. 2). In addition, mast cell infiltration and degranulation were observed under toluidine blue staining (Fig. 3). The difference between the EOM group A and the control group B was significant (p < 0.05).
(A) ET from group A presents with ciliated epithelial cell swelling, disordered and shedding cilia, thickened mucosa, and enlarged and expanded capillaries. (B) ET mucosal epithelium from group B revealed slightly swollen ciliated epithelium, with normal and neatly arranged cilia structure.
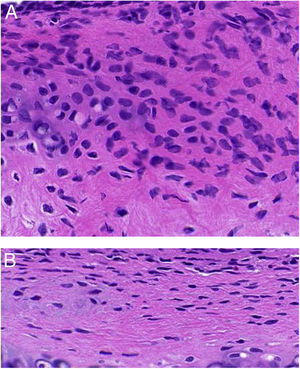
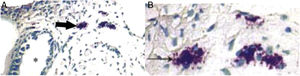
(A) Infiltration of mast cells in the tissue around the ET in group A. The black arrow indicates mast cells. The asterisk (*) corresponds to ET lumen (toluidine blue staining ×400). (B) Degranulation of active mast cells in the tissue around the ET in group A. Black arrow points to activated mast cells (toluidine blue stain ×1000).
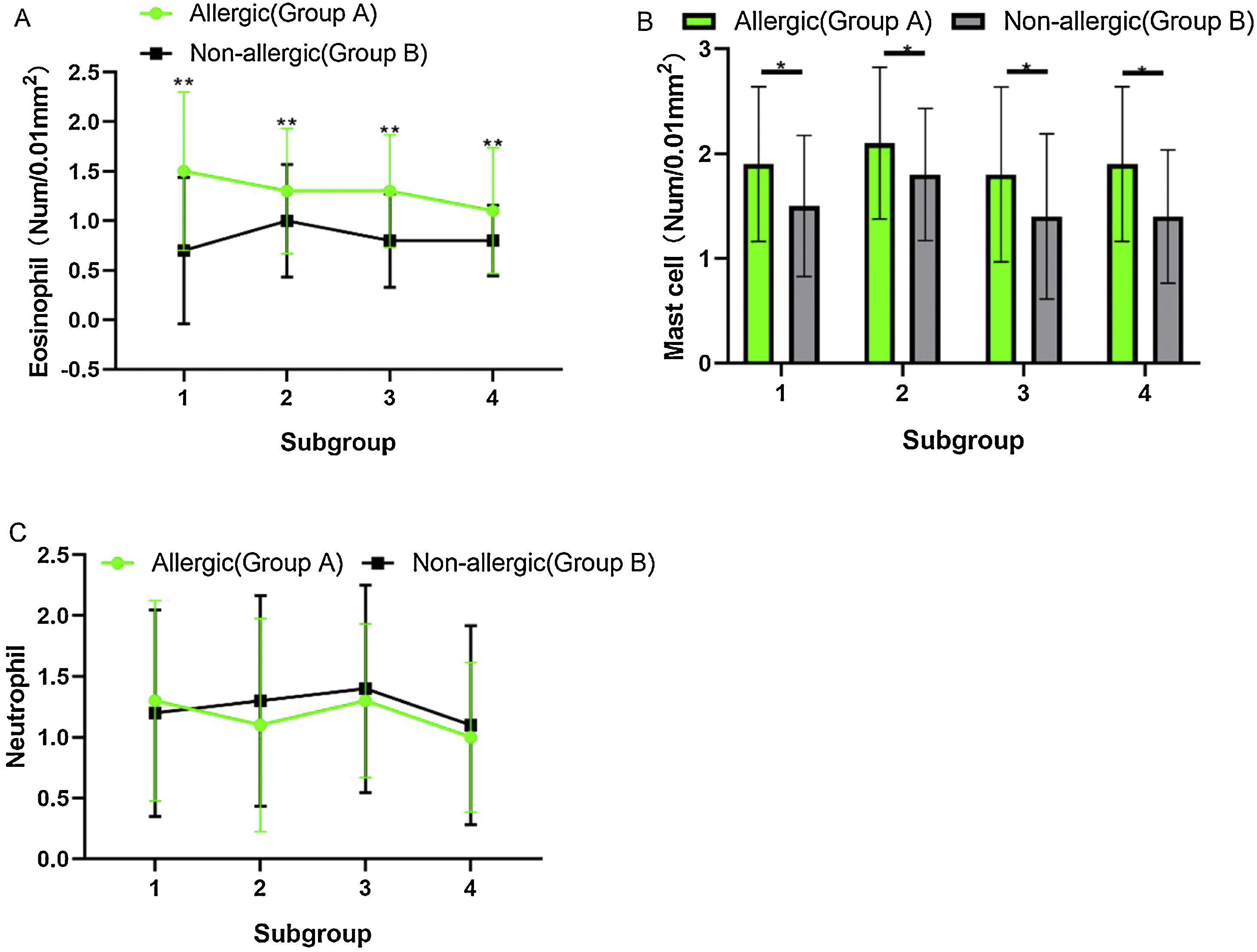
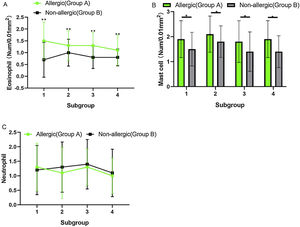
Counting of inflammatory cells under a high-power microscope (Fig. 4) showed no significant difference in eosinophils, mast cells, and neutrophils between subgroups A (1, 2, 3, and 4) or subgroups B (1, 2, 3, and 4) (p > 0.05). When the comparison was among subgroup A with subgroup B in 1, 2, 3, and 4, the counts of eosinophils and mast cells in group A were significantly higher than those in group B, and the difference was statistically significant (t-test, eosinophils: Fig. 4A, t = 4.608, η2 = 0.7797, p = 0.0037; mast cells: Fig. 4B, t = 3.520, η2 = 0.6737, p = 0.0125), but the count of the neutrophils was not significantly different between the two groups (Fig. 4C, p > 0.05).
(A) The counts of the eosinophils in group A were significantly higher than these in group B, and the difference was statistically significant (p < 0.05). (B) The number of mast cells in group A was significantly higher than that in group B, and the difference was statistically significant (p < 0.05). (C) The counts of the neutrophils did not differ significantly between the two groups.
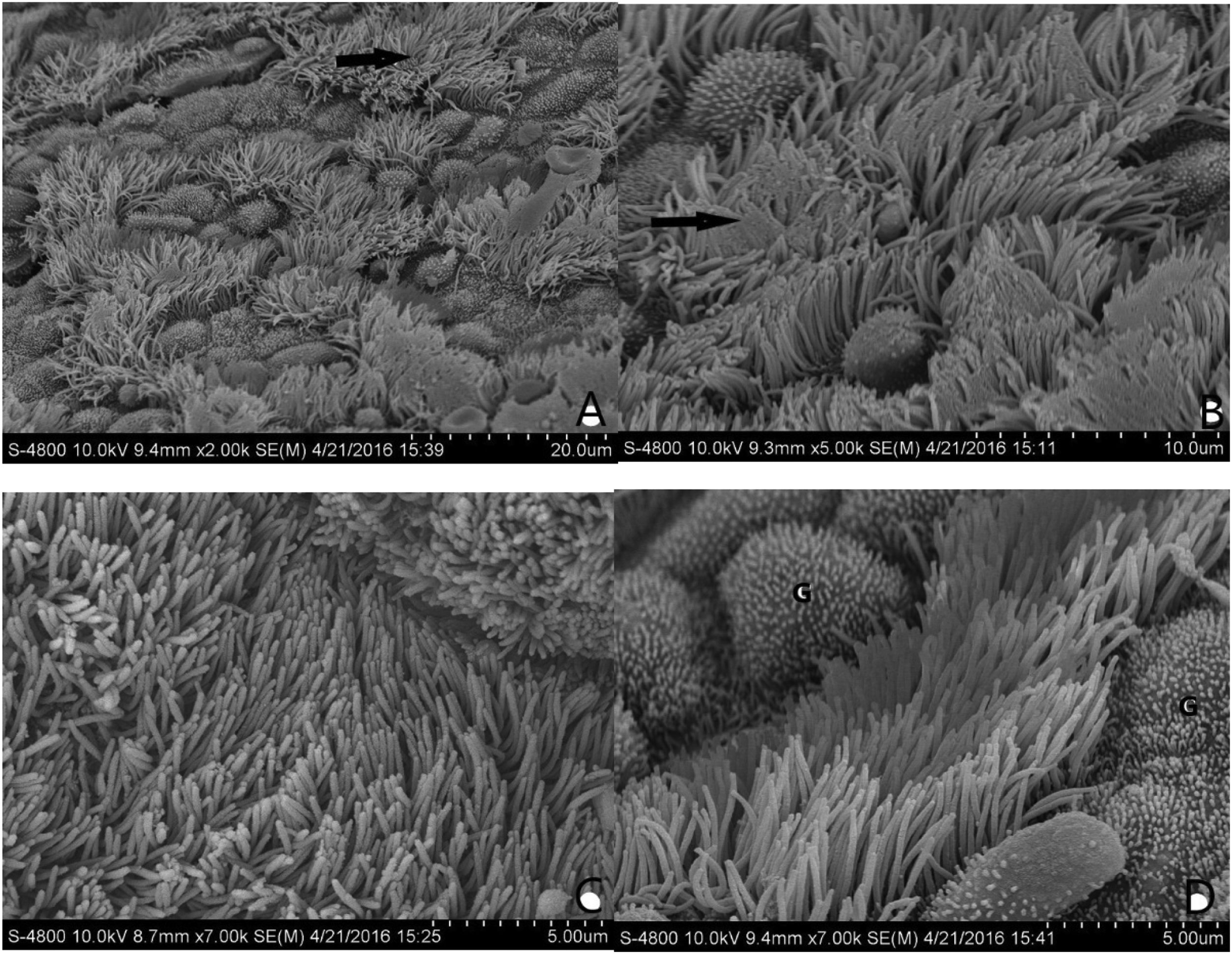
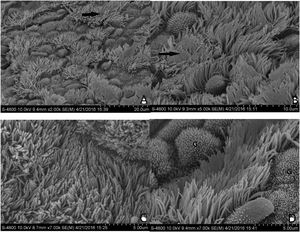
The normal mucosa of the ET under SEM in group B was covered with continuous cilia (Fig. 5C and D). However, the mucosa was discontinuous, with a large range of lodging or aggregation in group A (Fig. 5A and B). The differences between the two groups were significant.
(A and B) Morphological structure of the ET mucosa in group A under SEM. There is a significant and discontinuous loss of cilia in the ET mucosa. The number of ciliated cells in the opening area near the tympanic cavity is significantly reduced and the density decreased. In (A), the black arrow is directed toward the cilia missing area. In (B), the black arrow points to cilia obviously lodged, and a gathering area on a massive scale. (C and D) Morphological structure of the ET mucosa in group B under SEM. In (C), the full-length surface of the ET mucosa is covered with cilia, without ciliary loss or obvious lodging. In (D), the ciliated cells are scattered in the middle of the mucosa; The “G” indicates goblet cells.
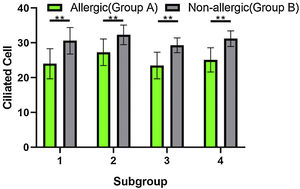
When the ciliated cells were counted under SEM, they showed no significant difference in their numbers among subgroups A1, A2, A3, and A4, or among subgroups B1, B2, B3, and B4 (p > 0.05). However, the numbers of ciliated cells in group A were significantly lower than those of group B (Fig. 6). The difference was statistically significant (t-test, t = 5.594, η2 = 0.8391, p = 0.0014).
DiscussionOME is a common disease with high morbidity in children and its pathophysiology is multifactorial, including allergy, bacteria, virus, gastroesophageal reflux disease, and ET dysfunction.27 Because the ET mucosa plays an important role in the excretion and maintenance of the micro-environment of the middle ear, ET dysfunction has gradually become a new point research largely enabled by the advancements in immunology and molecular biology.
Our study demonstrated that allergy was responsible for the significant changes in the pathology and morphology of ET mucosa. The morphology and cell composition of the eustachian mucosa were highly consistent with those of the respiratory mucosa.4 In order to further clarify the structural and functional changes of the ET mucosa in EOM, SD rats were used as the experimental carrier of the EOM model and the ET mucosa was observed under a microscope by HE staining and toluidine blue staining. The results showed that the numbers of eosinophils and mast cells in the ET mucosa of the EOM group were significantly increased compared with those in the control group (p < 0.05) and remained stable. This confirmed that the ET mucosa had an allergic reaction and would remain at a high level for a period of time in the EOM model induced by immunological pathogenesis. From this perspective, the clinical phenomena mentioned, where some patients with EOM do not exhibit mechanical obstruction of the ET, acute infection symptoms, or prolonged symptoms even after the acute phase passed, could now be explained.
In this experiment, it was observed that eosinophils and mast cells were increased in the EOM group, but neutrophils were not significantly different between the groups; this excluded infection factors and affirmed the reliability of molding, implying that the immune response plays an important role in the occurrence of EOM to a certain extent. Moreover, mast cell activation and degranulation were also observed under toluidine blue staining. A previous study showed that both eosinophils and mast cell activation could release various biologically active mediators, such as histamine, prostaglandins, and leukotrienes, which could cause tissue edema and increase transudate.28 Observation of ET mucosa and cilia swelling by HE staining revealed that mechanical obstruction caused by eustachian tube narrowing was one of the causes of EOM. The allergic inflammatory reaction occurring both in the middle ear mucosa and the ET mucosa increased the transudate of the middle ear cavity, which imposed the burden of evacuating fluid. Additionally, an increase of excretion in the ET caused further narrowing of ET, which raised the resistance to excretion. This could be considered as the mechanism of occurrence and development of EOM.
SEM has many observational advantages. Its image has a wide range of magnifications, from ten times to several hundred thousand times, and is presented at high resolution. The resolution ratio between the optical microscope and the transmission electron microscope is up to 3 nm and provides the necessary conditions to count the ciliated cells and observe micro changes. In this study, it was very important that a significant loss of cilia in the ET mucosa of the EOM group rats and a large range of cilia lodging or gathering was clearly observed. Moreover, the reduced scale of the number of effective ciliated cells was vital for comparison with that of the control group, which could be observed clearly under SEM.
In this study, the number of goblet cells was significantly increased. Undoubtedly, the reduction of ciliated cells and the abnormal ciliary morphology seriously affects the function of the middle ear effusion of the ET. In addition, the increase in eosinophils, mast cells, and goblet cells lead to increased mucus of the ET, causing blockage of the ET and further increasing the burden of fluid evacuation in the middle ear. Because the swing frequency of living cilia in the ET mucosa is greatly and easily affected by the environment and measurement methods, it is difficult to calculate the count of lodging and gathering of the ciliary cells. Combined with the ciliated edema observed in HE is staining and the obvious cilia gathering and lodging observed under SEM, the swing frequency of living cilia is also significantly reduced, which can have a negative effect on the ET mucosa.
Considering the limitations of this study, we next want to observe the living cilia and study the underlying molecular mechanisms, in order to provide objective evidence on the pathogenesis of EOM to be used for its prevention and treatment.
ConclusionThis study confirmed the role of ET dysfunction in the pathogenesis and development of EOM from the perspective of pathology and morphology caused by the allergy. In-depth study of the pathogenesis of EOM will assist in finding effective and feasible methods for the prevention and treatment of EOM, which are of great significance in accelerating disease recovery, reducing the economic pressure on patients, and improving the speech of preschool children.
FundingThis study was funded by a grant that Shouqin Zhao received from the National Natural Science Foundation of China (grant nº 81770989).
Conflicts of interestThe authors declare no conflicts of interest.
Peer Review under the responsibility of Associação Brasileira de Otorrinolaringologia e Cirurgia Cérvico-Facial.