Nasal septal abscess is a rare otorhinolaryngologic condition characterized by a collection of pus in the space between the cartilaginous or bony nasal septum and its overlying mucoperichondrium or mucoperiosteum.1 The majority of the nasal septal abscesses develop secondary to an infected septal hematoma, which most commonly occurs following nasal trauma.2 Other than septal hematoma, spreading of the microorganism from the infected sinonasal mucosa could result in an nasal septal abscess. Aerobic bacteria are the most common organisms isolated from nasal septal abscesses.2 Less commonly, anaerobic bacteria and fungi are isolated from nasal septal abscesses.3,4 To date, there have been no reports showing that the protozoans were isolated from a nasal septal abscess. This paper presents a case study of an adolescent male diagnosed with Entamoeba histolytica-isolated nasal septal abscess.
Case presentationA previously healthy 18-year-old male was admitted to the emergency department of a local hospital with complaints of abdominal pain, bloody diarrhea, and high-grade fever with chills of three days’ duration. No complaints of dysuria, cough, or sputum discoloration were present. In addition to these symptoms, the patient complained of nasal obstruction for a duration of three days, but reported no history of nasal trauma. The patient has a history of using contaminated water for personal cleaning and drinking. Based on patient history and clinical findings, the patient was diagnosed with amoebic dysentery and treated with oral ornidazole (500 mg) twice daily. The patient used topical decongestant (oxymetazoline) for nasal obstruction.
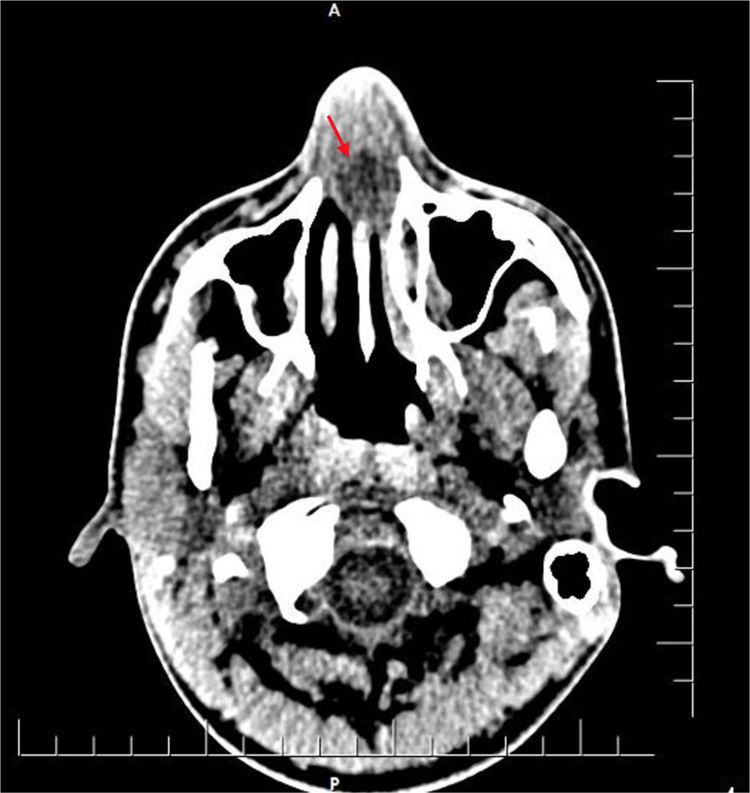
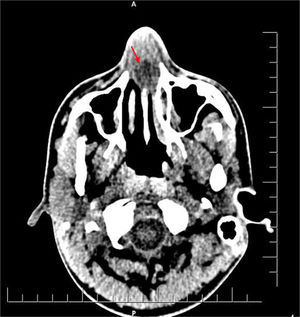
The patient was admitted to our hospital due to continuing complaints on the seventh day of the illness. A stool examination was performed by a gastroenterologist to confirm the diagnosis, at which point amoeba cysts were observed. ENT consultation was requested due to the nasal obstruction. The patient’s history indicated that he had been treated for a week due to amoebic dysentery and had been unable to breathe through his nose for a week. Although he used topical decongestants, his symptoms persisted. Anterior rhinoscopy revealed that bilaterally the nasal passages were totally obliterated, and there was fluctuating swelling in the septum. Computed tomography (CT) of the paranasal sinuses revealed a 1.8 × 1.8 cm bilateral collection of pus in the anterior nasal cavity indicative of septal abscess formation (Fig. 1).
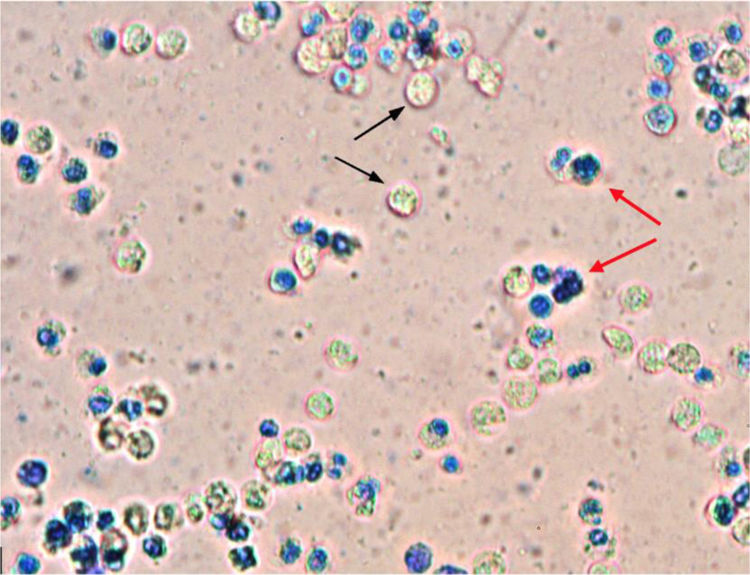
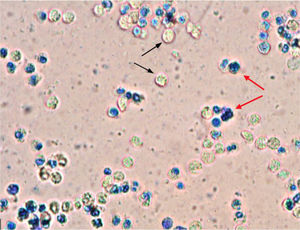
Abscess drainage was performed under local anesthesia and a Penrose drain was placed in the abscess cavity. After drainage, the nasal cavity was packed with Merocel (Medtronic Inc., Minneapolis, MN, USA). Amoeba cysts, Gram-negative bacilli, and abundant leukocytes were observed in pus samples drained from the nasal septum (Fig. 2). The Penrose drain and nasal packing were removed after two days, and the patient was treated with oral metronidazole 500 mg and ciprofloxacin 500 mg twice daily for two consecutive weeks. Followup at one week showed edematous septal mucosa, and there was no fluctuation or pus accumulation. One month later, the nasal septum was intact and there was no nasal deformity.
DiscussionNSA is a rare otorhinolaryngological condition, and its actual incidence remains unknown due to limited data. Bacterial colonization of the septal hematoma, which generally develops after nasal trauma, is the most common cause of NSAs. Although fighting, falling, and accidents are the common causes of nasal trauma, medical applications such as nasogastric tube placement and nasal intubation can also cause nasal trauma that results in a septal hematoma. Dental infections, paranasal sinus infections, nasal septal surgeries, nasal vestibulitis, and furunculosis can also lead to NSA formation. Aerobic bacteria such as Staphylococcus aureus, Haemophilus influenzae, and Streptococcus spp. are the most common causative agents of NSA.5 More rarely, Klebsiella pneumoniae, Enterobacteriaceae, S. milleri, and anaerobic bacteria are isolated from the NSA. Fungal NSA is rarely encountered and has only been reported in a few cases of immunocompromised patients.3 To date, no reports show that the protozoan species were isolated from the NSA. We are the first to report an NSA caused by the protozoan E. histolytica.
Amoebiasis, also known as amoebic dysentery, is an infection caused by E. histolytica. This infection occurs when mature cysts are ingested via food or water contaminated with feces containing amoebic cysts.6 Although most cases of amebiasis are asymptomatic, patients can present with cramping abdominal pain, watery or bloody diarrhea, and weight loss. The disease should be distinguished from other causes of abdominal pain, diarrhea, and weight loss, such as other infectious causes of gastroenteritis, including bacterial, viral, fungal, and parasitic pathogens, in addition to non-infectious causes, diverticulitis, inflammatory bowel disease and celiac disease. Amoebiasis is primarily an intestinal pathogen but can occasionally spread to extraintestinal sites such as the liver, brain, lungs, pericardium, pleura, and skin.7 The most common form of extraintestinal amoebiasis is amoebic liver abscess.8 This form of disease is caused by the trophozoite form of the E. histolytica, which invades intestinal mucosa and enters the portal venous system. The first treatment choice for uncomplicated amebic liver abscess includes pharmacologic therapy. Unlike pyogenic liver abscess, drainage is rarely required. Surgery may be necessary in case of fulminant colitis or peritoneal tissue perforations.
E. histolytica has not yet been confirmed in sinonasal mucosa or the septum. Extraintestinal manifestations of amoebic dysentery can occur along three routes: extension from the involved gastrointestinal tract, hematogenous spread from the primary site, or direct contact with contaminated food or water. In our report, the patient’s nasal obstruction complaint was concurrent with his gastrointestinal symptoms. This association suggests that contaminated water that caused gastrointestinal illness also contaminated the nasal mucosa via hair follicles or previously existing mucosal damage; however, hematogenous spread cannot be fully ruled out.
Patients with NSA generally present with nasal obstruction complaints. Less common symptoms include pain, nasal discharge, and fever. The primary complaint of our patient was nasal obstruction that persisted despite decongestant treatment. Bilateral, fluctuant, tender septal swelling was detected by anterior rhinoscopy.
Radiological confirmation of NSA is not mandatory. However, certain situations in which the underlying etiology remains unclear (e.g., spontaneous cases of immunocompromised patients) CT imaging may be required. CT imaging reveals both the leading cause of NSA in the absence of trauma (e.g., paranasal sinus, dental infections) and potential complications of NSA, such as orbital complications. Since our patient did not have a history of nasal trauma or surgery, a CT scan was performed that revealed minimal mucosal thickening in the paranasal sinuses and bilateral collection of pus in the anterior nasal cavity.
The treatment of NSA usually consists of abscess drainage to prevent complications and to remove pressure on the septum to avoid damaging the nasal septal cartilage. Once the drainage is complete, a sample of pus should be sent for microbiological assessment, which should involve routine nasal culture and an active search for fungi and other atypical pathogens. Empirical antibiotic treatment should be initiated immediately until the causative pathogen is identified. Amoeba cysts, Gram-negative bacilli and abundant leukocytes were observed in pus samples drained from the NSA of our patient. The patient was treated for two weeks with oral metronidazole for E. histolytica and ciprofloxacin for Gram-negative bacilli. No recurrence or nasal septal deformities were observed at followup.
ConclusionNSA is a rare pathology that can be overcome without serious complications by drainage and medical treatment, if detected early. It is important to note that the pathological agent is identified by detailed microbiological evaluation and treated accordingly. Especially in patients with no history of nasal trauma, atypical etiological agents should be evaluated with extra caution. Although E. histolytica has not previously been reported among the agents causing NSA, it should be kept in mind in patients with gastrointestinal complaints or with suspected contaminated food and water ingestion or contact.
Conflicts of interestThe authors declare no conflicts of interest.
Peer Review under the responsibility of Associação Brasileira de Otorrinolaringologia e Cirurgia Cérvico-Facial.