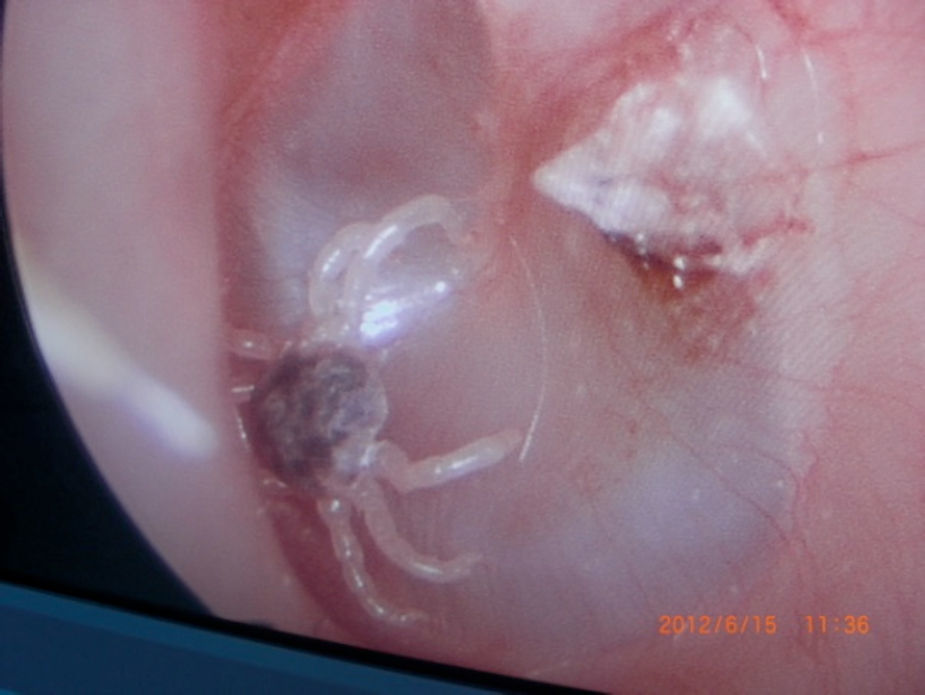
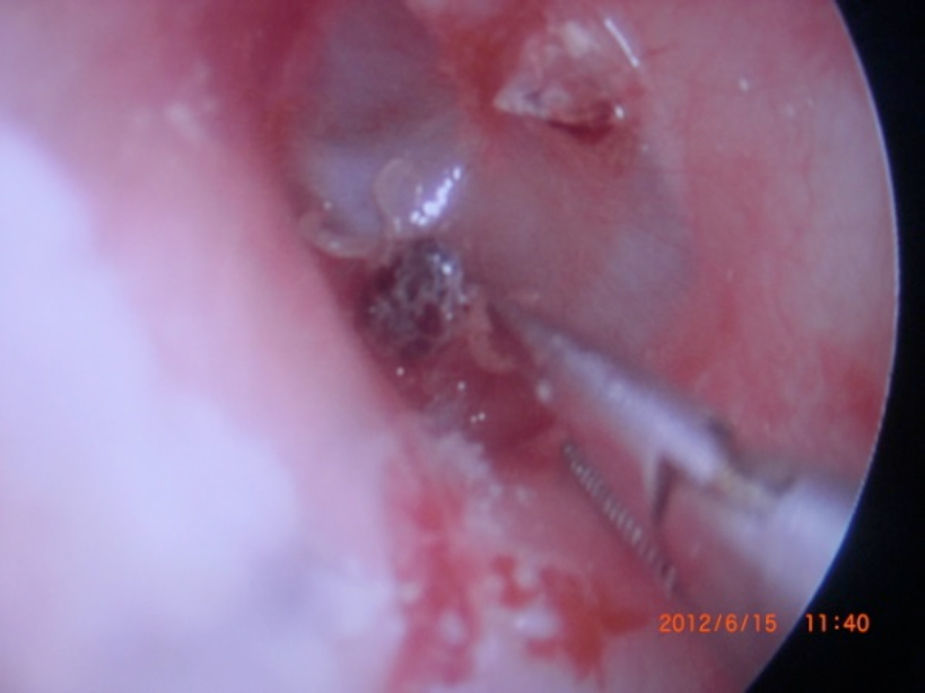
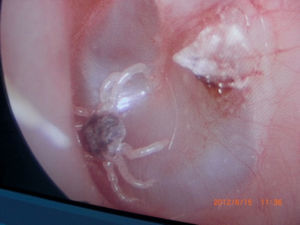
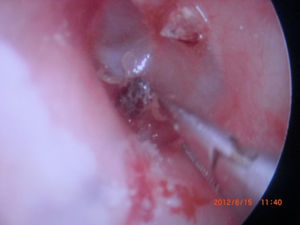
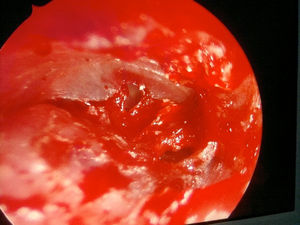
Otoacariasis, the attachment of ticks and mites within the ear canal is a common phenomenon especially in rural areas.
ObjectiveTo determine the clinical and demographic features of cases with detected ticks in the ear canal, which is a common health problem, and identify tick species.
MethodsData of patients who had otoacariasis were collected. We also investigated all ticks at the Veterinary Department of Kafkas University.
ResultsWe present the data of patients with otoacariasis. All ticks were identified as otobius. Otobius ticks were found not related with any complications.
ConclusionIt is very important to detect ticks in the ear canal as they act as vector of some diseases. Identifying species of ticks may help clinicians to prevent further complications associated with vector-borne diseases.
A otoacaríase, fixação de carrapatos duros e moles no interior do conduto auditivo, é fenômeno comum, especialmente em áreas rurais.
ObjetivoDeterminar as características clínicas e demográficas de casos de carrapatos detectados no conduto auditivo externo, um problema de saúde frequente, e identificar as espécies do ácaro.
MétodoColetaram-se dados dos pacientes com otoacaríase, e todos os carrapatos foram investigados no Departamento de Veterinária da Universidade Kafkas.
ResultadosOs dados de pacientes com otoacaríase são apresentados. Todos os carrapatos foram identificados como pertencentes ao gênero Otobius e constatou-se não haver relação entre os carrapatos e qualquer tipo de complicação.
ConclusãoÉ muito importante detectar carrapatos no conduto auditivo externo, pois esses ácaros funcionam como vetores para algumas doenças. A identificação da espécie do ácaro pode ajudar o clínico a prevenir complicações associadas às doenças transmitidas por esse vetor.
Otoacariasis, the attachment of ticks and mites within the ear canal, is a common phenomenon in livestock and domestic animals, where the ears are a common site of attachment of mites, such as Psoroptes and Otodectes, and ticks, such as Otobius megnini and several Rhipicephalus and Hyalomma spp.1–3
Ticks have existed for thousands of years on Earth. There have been ticks in the environment where humans and animals have existed. There are over 900 different tick species throughout the world, and approximately 46 of these species have been found to be active in Turkey. Ticks act as a vector of infection of viral, bacterial, rickettsial and parasitic infections, spreading through both mechanical and biological pathways. Therefore, they are accepted as a public health problem all over the world.4,5
Approximately 80% of the world's tick fauna are ixodid ticks (hard ticks) and the rest are argasid ticks (soft ticks) out of over 900 currently known tick species. The world's argasid tick fauna is divided into four genera, including Argas, Carios, Ornithodoros and Otobius.6
Most ticks have a preference for feeding on certain groups of wild animals, with some even being quite host specific, whereas the number of species pertinent to domestic animals and/or humans is limited. Few species adapt to livestock or feed on a human subject, and they develop into efficient vectors of a range of pathogenic microorganisms. All human tick-borne diseases are zoonoses.7
Most people become infected in spring and summer, and especially by tick bites. However, there are various diseases caused by ticks; some of them need special interest. Rocky Mountain fever, tularemia, Lyme disease and Crimean-congo hemorrhagic fever (CCHF) need more attention because of their higher incidence, morbidity and even mortality.
CCHF is one of the most important tick-borne viral diseases of humans, causing sporadic cases or outbreaks of severe illness across a huge geographic area. Hyalomma ticks are the principal source of human infections. Nairovirus in the family Bunyaviridae is the etiologic factor of CCHF. Tularemeia is a multi-systemic disease caused by the bacterial pathogen Francisella tularensis. Biting flies, water exposure, food and aerosols as well as ticks can transmit the disease. F. tularensis is highly infectious and clinical infection may be seen after exposure to very few amounts of bacteria. Lyme disease is caused by the spirochete Borrelia burgdorferi. The main vectors for Lyme disease are ticks. Mouse and small mammals are natural reservoirs for B. burgdorferi. Rocky Mountain spotted fever is caused by Rickettsia rickettsii. Ticks are the main vector and the disease is more common from April to September.
The main complications of intra-aural foreign bodies are canal abrasion, laceration or bleeding, but these can also cause otitis externa, perforate or rupture the tympanum, suppurative otitis media, affecting the middle ear.8 In our clinical practice, cases of human otoacariasis were a common occurrence at an ear, nose and throat (ENT) ward over a long period, and this prompted a prospective etiological and epidemiological study, the results of which are reported herein. The aim of this article is to report demographic and clinical characteristics of the cases of ticks in the ear canal that we have determined almost in contrast with literature as well as reviews in the literature.
MethodEthical considerationsFor ethical considerations, an application was made to the local University Ethics Committee. The Ethics Committee approved the admission on 27 September 2012 with decision number 16 (Supplement 1).
The patients who were diagnosed with ticks in the ear canal in the emergency department and otorhinolaryngology department of our State Hospital between January and December 2012 have been included the study. Our state (land area 5576km2; population 105,000; elevation 1900m; mean annual rainfall 500mm; mean annual temperature 13°C; mean relative humidity 73%) is located in northeast of our country. The main economic activities in the district are agriculture and livestock.
Patients’ age, gender, occupation, the region where they live in, application time, complaints, physical examination findings, results of consultation with the infectious disease department and follow-up (at least ten days) have been recorded.
The cases were treated with the same intervention of removing the ticks with alligator forceps. The procedure was carried out with otoendoscopy or otomicroscopy to avoid any harmful effects to ear canal. Otoendoscopic and microscopic evaluation was made to check tympanic membrane and outer ear canal in every patient after the removal of the ticks.
The ticks removed from the external ear canal of the patients were preserved in alcohol and stored in separate, labeled vials. Infectious disease consultation was made for every patient. We could not make any tests for possible infectious diseases because of insufficient conditions in our hospital. Ticks were kept for investigation in terms of possible tests for contagious diseases.
Ticks were identified using standard keys and descriptions. Reference collections from a larger study on tick ecology that included voucher specimens of the species identified herein are deposited at the Institute of Veterinary of our state university. Of a total of 31 samples collected, all had accompanying patient details such as age, sex and the other domestic livestock present in their households. The analysis of tick species was carried out for the entire 31 cases.
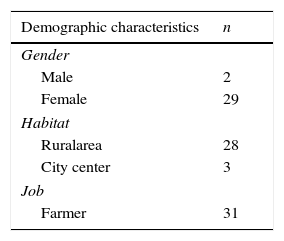
ResultsThere were 29 female and 2 male patients. Ages of the patients vary from 17 years of age to 72 years of age with an average of 32.28 years of age. The source of income of all patients was agriculture and livestock, and 28 of them lived in the rural area, whereas 3 lived in the city center (Table 1).
The time of the applications was also analyzed and presented in Table 2. There was a significant increase in the peak during September/May.
The complaints of the administration have been illustrated in Table 3.
In the physical examination, the ticks were found on 17 patients’ right ear and on 14 patients’ left ear out of 31 patients. Ticks were removed from the outer ear canal of 27 patients, 3 ticks were removed from the tympanic membrane and one was removed from the middle ear of a patient who had chronic otitis media concurrently. In one patient two ticks were removed at the same time. Also two ticks were removed from different ears of the same patient at different times.
Outer ear canal edema and hyperemia were seen in all patients. Removal of ticks was carried out with alligator forceps, using local anesthesia. Otoendoscopy or otomicroscopy was used in all cases and control was made with microscopic evaluation.
No complication such as outer ear canal laceration or tympanic membrane injury on the patients who had outer ear canal ticks has been determined. There were hyperemia and milimetric to small tympanic membrane perforation in 3 patients from whom the removal from the tympanic membrane was made. One patient had permanent tympanic membrane perforation whereas two of the patients’ perforation was closed spontaneously. No complication resulting from patient having the tick removed from middle ear was observed.
Infectious disease consultation was made in all cases in order to avoid diseases hosted by ticks. Patients were controlled especially for crimean-congo hemorrhagic fever and tularemia. No sign, symptom and finding of these diseases were observed, although tularemia is not rare in the region. Even crimean-congo hemorrhagic fever was not observed in our town in any patients, who were checked for both diseases.
Patients were also controlled in our ambulatory service on the second and the tenth day. Except for one permanent perforation, no other complication was observed in the follow up of the patients.
All ticks were analyzed by the Veterinary Department of our regional University. All of the ticks were reported as Otobius species (Figs. 1–4).
DiscussionTicks and tick-borne diseases both affect animals and humans and have a significant cost. Approximately 10% of over 900 known tick species act as a vector of different pathogens of animals and humans, besides being responsible for direct damage due to their feeding behavior.4
Type analysis wasn’t made at the time of the administration. The ticks removed from the external ear canal of the patients were preserved in alcohol, and stored in separate, labeled vials. Ticks were collected for both possible diseases that can develop with time and for the analysis of possible hosting diseases. Out of a total of 31 samples collected, all had accompanying patient details such as age, sex and the other domestic livestock present in their households. Reference collections from a larger study on tick ecology that included voucher specimens of the species identified herein are deposited at the Institute of Veterinary in our state university. The analysis of tick species was carried out for the entire 31 cases and identified as Otobius Megnini.
O. Megnini has a single-host lifecycle consisting of larval stages followed by a variable number of nymphal stages. The parasitic phase may exceed 200 days. The last nymphal stage drops to the soil to transform into the non-parasitic adult stage. Fertilized female O. Megnini lay several batches of eggs.
Larvae and nymphs of the spinose ear tick Otobius Megnini can be found in the external ear canals of livestock, companion animals and occasionally humans and may cause otitis. The spinose ear tick Otobius Magnia is an American species that has been widely disseminated by international shipments of infested domestic animals. This tick occurs in the western parts of the USA and some parts of South America, as well as in the Afrotropical and Oriental regions.
The larvae and nymphs attack a wide range of domestic and wild animals. The immature stages of otobius are parasitic for long periods in the external ear canals of their hosts. Adult otobius are non-parasitic and this means that they do not feed. Heavy infestations of these picks may occur on horses, cattle, sheep and dogs.
Without acting as vectors of disease, ticks can be harmful to livestock and are of great economic importance simply because of their direct effects. O. Megnini can cause otitis in humans, may play a role in the maintenance of the agent of Q fever in nature and may be associated with paralysis in children.9,10
A variety of microorganisms including protozoa, rickettsiae, spirochaetes and viruses can be transmitted from animals to humans through ticks. Moreover, ticks can cause severe toxic conditions such as paralysis and toxicosis, irritation and allergy.7
Tick-borne diseases are still ranked high in terms of their impact on the livelihood of resource of poor farming communities in developing countries. Global economic importance of ticks is particularly high for livestock.
Transfer of a disease starts with the feeding of a tick on an infectious vertebrate host. During feeding they take the pathogen through blood meal. They transfer pathogen to another host when feeding again.11 They can also result in paralysis, toxicosis, severe local reactions such as forms of pruritus, secondary bacterial infections and a variety of reactions caused by immune-mediated responses.12
Tick bites are commonly seen in the head and neck region, in the lower extremity and in the arms. Occupation groups and age distribution are reported in a very wide range. There were reports of different ways of coming across with ticks, such as during agriculture and livestock activities as in our cases and also during picnics in the environment or close contact with animals, like people working in butcheries or working as veterinaries.
There was a dear peak during May/September, when the animals were feeding outside during better air conditions in the region, as August is the month with the highest number of cases, relative to much more exposure. It was reported that the ticks are increasing in hot seasons and also complaints usually are made between June and September, when the risk of exposure is very high.13,14
Patient with exposure history and symptoms including fever, malasia and other nonspecific symptoms, together with physical findings suggestive of vascular leak and coagulation defect must be evaluated for CCHF. Leukopenia, thrombocytopenia and elevated serum liver enzymes may be seen in laboratory evaluation. Certain diagnosis is made by showing viral nucleotide and viral anticores. Deaths related especially with CCHF have increasingly been reported in the last few years. Different treatment options including general supportive measures, antiviral therapy, antibody therapy and vaccines are used in CCHF.15
Different forms of tularemia, including ulceroglanduler, oculoglandular, pneumonic, oropharyngeal, gastrointestinal, and thphoidal forms may be seen in clinical practice. The most common type is ulceroglanduler form, characterized by chills, fever, head and muscle pain and prostration, usually 3–6 days after exposure. Prompt antibiotic treatment, such as fluoroquinolones, aminoglycosides and supportive therapy, generally has good results, with favorable prognosis. Also, different vaccines for tularemia have been developed.16
First stage of Lyme disease is seen 7–10 days after exposure. A typical rash called erytema migrans occurs at the side of the tick bite. Also, fever, fatigue, arthralgias, headaches, cough and lymphandenopathy may be seen. In stage 2 of Lyme disease, also called early disseminated stage, multiple secondary cutaneous annular lesions, fever, adenopathy and central nervous system symptoms may occur. In stage 3, late chronic disease, symptoms include chronic arthritis, central nervous system problems, dermatitis and keratitis. Laboratory tests, including ELISA tests, help the diagnosis of Lyme disease. In most cases antibiotic treatment, including doxycycline or amoxicillin, is curative.17
First symptoms usually occur 5–7 days after exposure in Rocky Mountain spotted fever. Malaise, myalgia, fever headaches, nausea and vomiting are common symptoms. With non-specific symptoms, an exanthem appears within the first few days of symptoms. The skin lesions may change and coalesce to form large areas of ecchymosis and ulceration. Respiratory, circulatory and neurologic complications may occur. Patients with glucose-6-phosphate dehydrogenase deficiency are at high risk for complications. Diagnosis of Rocky Mountain spotted fever is based on clinical signs and symptoms. Laboratory tests, especially immunofluorescent staining for rickettsia, help the diagnosis of Rocky Mountain spotted fever. Tetracycline, doxycycline, chloramphenicol and fluoroquinolones are used in the treatment of Rocky Mountain spotted fever. Prompt antibiotic treatment helps good prognosis.18
Infectious disease consultation was made in every patient, and no diseases related with ticks were observed in the cases. This was attributed to the presence of different types of ticks. Hosting and contaminating properties change with the type of ticks. Geographic regions also affect the type of ticks.19,20
Although many of the patients reported with foreign bodies in the ear canal are under 8 years old, in the report that we present the youngest case where ticks in the outer ear were found was a 17 year-old individual. In otorhinolaryngology practice, child patients comprise many of the cases of foreign bodies in the ear canal or the nasal cavity; however, in adults, foreign bodies in the ear are usually caused by trauma or accidents.
The main symptom of foreign bodies in the ear canal is pain. Sometimes there is no symptom related with foreign bodies. Families can rarely see or suspect foreign bodies, which may be the administration symptom.21 We observed in our cases symptoms such as fullness in ear, hearing loss, pain, discharge in the ear and tinnitus. In these cases, we also observed that the pain usually starts approximately 3 or 4 days after fullness and hearing loss starts; therefore, administration to our department is made generally 3 or 4 days after the entrance of ticks. When we analyzed the complaints in detail, we saw that the patients usually seek further examination when pain accompanies other symptoms. We also demonstrated that hearing loss in patients was caused by the obstructing mass effect of ticks.
It was reported that removal of foreign bodies done with general anesthesia in some cases.22 This necessity may be due to dissonance of children to examinations or interventions. In our cases all of the patients were adults and none of them needed general anesthesia.
In literature it was highlighted that tick removal is suggested to be carried out with mechanical methods instead of chemical ones.23 We also tried as much mechanical removal as possible by pulling out the head of ticks with the help of alligator forceps, in order to extract the tick alive in the cases of ticks in the ear canal.
The impact of tick feeding and tick-transmitted diseases and their control should be much better defined in economic terms. Tick control on livestock remains, to a large extent, based on acaricides, but their use in possible combination with anti-tick vaccines and utilization of host resistance to ticks should reduce dependency on chemical tick control. Research into novel, ecologically sound, practical tick control methods should be intensified, and implementation of existing methods to vaccinate against tick-borne diseases is recommended, as well as intensified research toward developing novel vaccines and delivery systems. Much progress is expected in the next few years through the use of tools available in the post-genomic era. It can also be expected that new tick-borne pathogens will continue to be discovered. Furthermore, in the future, we may be able to exploit for medical purposes some of the considerable number of bioactive molecules of the fascinating, largely undisclosed tick pharmacy.
These cases are of interest in that O. Megnini infestations seem to produce little annoyance to humans during the extended larval and nymphal developmental periods. In all the cases, the ticks had molted into nymphs and were completely engorged when removed.
It is evident that human parasitism by this tick is a potential health problem in rural areas of our country, where people live in close contact with domestic animals. O. Megnini infestations could result in ear damage or the transmission of infectious disease agents.
ConclusionIn conclusion, it has to be kept in mind that ticks in cases with foreign bodies in the ear canal are seen especially in rural areas. In contrast with other foreign bodies, they can result in systemic symptoms and problems. Therefore, patients must be evaluated systematically, even in consultations with the related departments. Hosting and contaminating are very important for both the patients’ prognosis and the physicians’ health. After coming across with patients with ticks on their bodies, to inform the cases to related units to receive the necessary education and interferences is important for public health.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Gökdoğan O, Çakabay T, Baran H, Karabulut B, Tasdemir C, Vatansever Z. Otoacariasis: demographic and clinical outcomes of patients with ticks in the ear canal. Braz J Otorhinolaryngol. 2016;82:416–21.