To compare the efficacy of endoscopic and open resection of sinonasal malignancies.
MethodsThe search was performed using PubMed (1950–2020), Embase (1974–2020), the Cochrane library, and the website clinicaltrials.gov. The hazard ratio, HR, 95% confidence interval, CI, of the rates of overall survival and disease-free survival and the demographic characteristics of the included studies were extracted and analyzed. Pooled analysis was conducted with the studies’ individual patient data, using log-rank test, Kaplan–Meier survival, and Cox regression analysis.
ResultsOf 1939 articles retrieved, 23 articles were included. Overall, 1373 cases were incorporated into the final analysis, 653 (47.56%) of which underwent the surgery through an endoscopic approach, whereas 720 (52.44%) cases utilized the open approach. The overall survival was comparable between endoscopic and open resection (HR = 0.84 [95% CI: 0.65–1.07], p = 0.16; random effects analysis). Pooled analysis with Cox regression revealed signifcant differences in overall survival (HR = 0.568 [95%CI:0.380-0.849], p = 0.006) and disease-free survival (HR = 0.628 [95%CI:0.424-0.929], p = 0.02) between endoscopic and open approaches.
ConclusionThe aggregated evidence suggests the survival outcome of endoscopic resection is comparable or greater than that of open resection of sinonasal malignancies.
The concept of endoscopic endonasal surgery was first proposed in 1986 to deal with recurring rhinosinusitis.1 This approach had advantages such as better intraoperative vision, shorter recovery time, and potentially smaller postoperative facial scar or deformity.2 Sinonasal malignancies are known to be rare and carry a high risk of mortality. In 2000, Goffart applied endoscopic resection (ER) for the treatment of selected malignant sinonasal tumors, as he observed that there was little difference in the recurrence rate of benign lesions.3 Since then, endoscopy has been utilized in the treatment of sinonasal malignancies. However, it is yet to be discussed whether progressive margin resection, uncontrolled intraoperative hemorrhage, and the difficulty in skull base reconstruction, all of which occur in endoscopic resection, can increase the risk of mortality of the disease,4 especially advanced tumors. Meanwhile, with the development of high-definition endoscopy technology, the superiority achieved in implementation of endoscopy in malignancies cannot be neglected. Several meta-analyses have compared the outcome and efficacy of the endoscopic and open approaches in sinonasal malignancies indirectly, drawing a conclusion that the two approaches were comparable.5,6 In a recent study, Lu arrived at a conclusion that the length of hospitalization was shorter in endoscopic endonasal surgery than in open resection (OR).7 In another meta-analysis, Hur demonstrated that endoscopic resection of sinonasal melanoma has better overall survival.8 However, due to the low incidence of sinonasal malignancies, the selection of the chosen surgical procedure in sinonasal malignancies is still to be discussed. The evidence-based implementation of endoscopic and open approaches remains to be explored due to the rarity and heterogeneity of sinonasal malignancies.
The purpose of our study was to conduct a meta-analysis of the current literature to compare the outcome of sinonasal malignancies via endoscopic and open approaches and to determine whether and when endoscopic approaches could achieve a comparable or better efficacy.
MethodsSearch strategyThis systematic review and meta-analysis were conducted and reported based on the MOOSE (Meta-analysis of Observational Studies in Epidemiology) guidelines9 since all the trials involved were observational studies.
The search was performed using PubMed (1950–2020), Embase (1974–2020), the Cochrane library, and the website clinicaltrials.gov by two reviewers. The keywords used in the searching strategies included “sinonasal”, “malignancy”, “endoscopic”, and Medical Subject Headings (MeSH) terms, combined by Boolean operators. We retrieved literature from the reference lists of the obtained literature and contacted the authors by e-mail to include all the available studies.
Inclusion and exclusion criteriaThe following inclusion criteria were identified systematically in all the included studies: 1) The participants were diagnosed with sinonasal malignancies pathologically; 2) The participants received surgery with a curative intention and were allocated to the ER group (including endoscopic endonasal surgery and endoscopic-assisted surgery) or the OR group based on the surgical approach employed. Cases in each group were no less than 3 individuals; and 3) The hazard ratio (HR) and 95% confidence interval (CI) of the overall survival (OS) or disease-free survival (DFS) in each study were provided or could be calculated. Studies meeting the following criteria were excluded: 1) Tumor had not primarily originated from the nasal sinuses; and 2) Follow-up time was less than 12 months. Studies were included in a pooled-analysis when individual patient data were provided.
Data extraction and statistical methodThe HR and 95% CI of the rates of OS and DFS along with the demographic data including age, sex, diagnosis, stage of disease, statement of previous treatment, adjuvant therapy, and number of participants in each group were extracted from the included studies and aggregated by the reviewers independently. The HR and standard error (SE) were calculated using the methodology described by Tierney et al.10 when only the number of patients randomized into each arm of the trial, total number of events, and p-values of the log-rank test were provided. We also extracted data from Kaplan–Meier curves by tracing via the Engauge Digitizer software (version 12.1, free software downloaded from https://github.com/markummitchell/engauge-digitizer). Meta-analysis was conducted on the Review Manager software (version 5.3, free software downloaded from https://training.cochrane.org/online-learning/core-software-cochrane-reviews). Subgroup analyses based on previous treatment, pathology type, and comparability of studies were performed. When individual patient data were provided, a directive comparison was conducted using the SPSS software (version 23.0.0.0, IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp). We categorized Kadish A/B and American Joint Committee on Cancer (AJCC) stages T1/T2 into “low stage”, and Kadish C/D and AJCC stages T3/T4 into “high stage”.5 The categorical variables were compared using a Chi-square test, whereas the continuous variables were compared using the Student’s t-test or Mann–Whitney U-test. Survival outcomes of both groups were compared using the Kaplan–Meier method, log-rank test, and the Cox regression analysis. A p-value of 0.05 or less was considered significant.
Bias and quality assessmentQuality assessment for each study was evaluated using the Newcastle-Ottawa scale (NOS).11 The quality of evidence for each outcome was rated via Grading of Recommendations, Assessment, Development, and Evaluations (GRADE).12
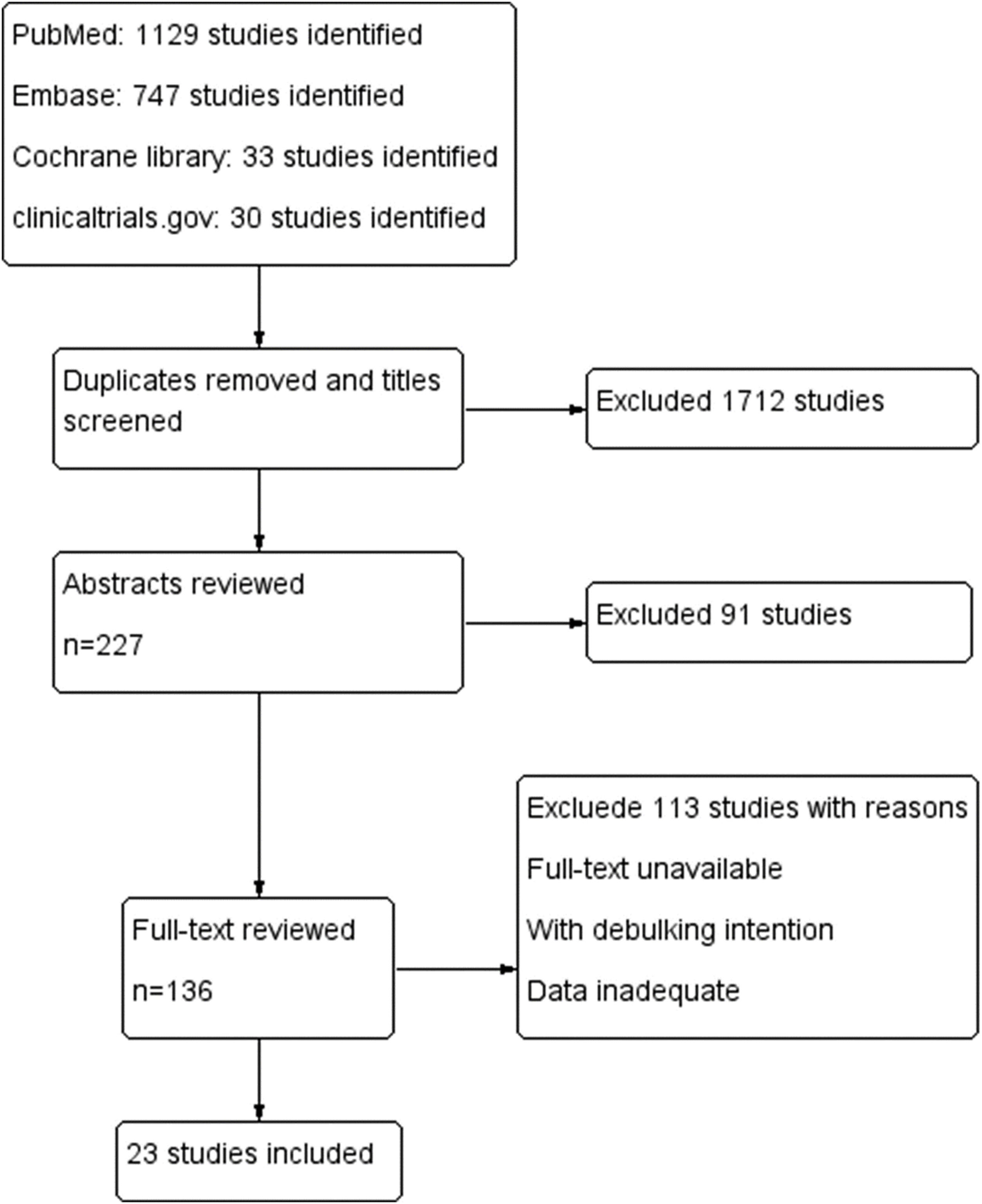
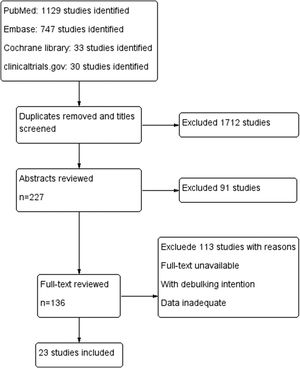
ResultsA total of 1939 articles were retrieved based on our search strategy. Of these, 227 articles were reserved after screening the title and removing the duplicates. After reviewing the abstracts, full-text analysis was carried out in 136 articles, according to the inclusion and exclusion criteria. Finally, 23 articles (Fig. 1) were included in the final meta-analysis, the characteristics of which are summarized in Table 1.13–35
Studies included for meta-analysis.
| Study | Year | Study type | Study period | Country | Nº of cases | Diagnosis (ER/OR) | Previous treatment | Adjuvant therapy | Mean age (y) | Nº of male | FU, mo | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | ER | OR | ER | OR | ER | OR | |||||||||
| Constantinidis, J. | 2004 | RC | 1975–2000 | Germany | 23 | 11 | 12 | ON | NA | 8 | 9 | 51 | NA | 102.55 | 77.36 |
| Orvidas, Laura J. | 2005 | RC | 1980–2001 | USA | 23 | 3 | 20 | AC | NA | 2 | 20 | 71.33 | 17 | 36.33 | 73.87 |
| Roth, T. N. | 2010 | RC | 1992–2007 | Switzerland | 19 | 13 | 6 | SNMM | NA | 5 | 5 | 67.30 | NA | 46.46 | 28.50 |
| Lund, V. J. | 2012 | RC | 1963–2010 | UK | 109 | 31 | 78 | SNMM | NA | NA | NA | NA | NA | NA | NA |
| Song, C. M. | 2012 | RC | 1989–2008 | South Korea | 28 | 16 | 12 | ON | NA | 21 | 12 | NA | NA | NA | NA |
| Guo, L. | 2014 | RC | 1994–2011 | China | 23 | 8 | 15 | CS (6/14), MYCS (1/0), MECS (1/1) | 5 | NA | NA | 26.78 | 9 | 51.63 | 77.62 |
| Saedi, B. | 2014 | RC | 1999–2010 | Iran | 160 | 72 | 88 | SCC (5/25), ACC (8/11), SNUC (4/9), ON (15/9), SNMM (21/8), ewing sarcoma (5/8), rhabdomyosarcoma (0/3), Sarcoma (0/3), transitional cell carcinoma (0/3), others (11/5) | 0 | 38 | 65 | 47.60 | 112 | 22.00 | 20.00 |
| Swegal, W. | 2014 | RC | 1998–2012 | USA | 25 | 12 | 13 | SNMM | 6 | 11 | 11 | 65.50 | 14 | 32.40 | 46.80 |
| Grosjean, R. | 2015 | RC | 1998–2009 | France | 74 | 43 | 31 | AC | 0 | NA | 32 | 69.20 | 72 | 44.40 | 57.60 |
| Ledderose, G. J. | 2015 | RC | 2000–2010 | Germany | 22 | 12 | 10 | SNMM | 22 | 12 | 10 | NA | NA | NA | NA |
| Won, T. B. | 2015 | RC | 1994–2013 | South Korea | 133 | 70 | 63 | SNMM | 0 | NA | NA | NA | NA | NA | NA |
| Cao, W. | 2017 | RC | 1995–2014 | China | 33 | 15 | 18 | SNMM | 0 | 17 | 18 | 65.40 | 17 | 42.00 | 49.20 |
| Hagemann, J. | 2019 | RC | 1993–2015 | Germany | 225 | 123 | 102 | SCC (51/52), AC (16/18), SNMM (17/11), ON (8/5), ACC (7/3), lymphomaa (6/0), sarcoma (7/4), SNUC (3/6), others (8/3) | NA | 57 | 73 | NA | 135 | 54.40 | 45.40 |
| Yin, G. | 2019 | RC | 2004–2016 | China | 54 | 27 | 27 | SNMM | 0 | 20 | 13 | 57.07 | 28 | 28.37 | 25.33 |
| Lai, Y. | 2020 | RC | 2000–2016 | China | 92 | 57 | 35 | SNMM | 0 | 45 | 25 | 65.00 | 52 | 30.72 | 21.60 |
| Lee, G. | 2017 | RC | 1999–2015 | South Korea | 31 | 16 | 15 | SNMM | 0 | 12 | 13 | NA | 18 | NA | NA |
| Batra, P. S. | 2005 | RC | 1995–2003 | USA | 24 | 9 | 15 | ON (0/8), SCC (2/5), AC (2/1), SNMM (2/0), SNUC (1/0), adenosquamous carcinoma (1/0), Sarcoma (1/1) | NA | 12 | 18 | NA | NA | NA | NA |
| Eloy, J. A. | 2009 | RC | 1997–2006 | USA | 66 | 18 | 48 | SCC (0/25), ON (10/4), ACC (3/8), AC (0/4), SNUC (1/2), SNMM (0/2), hemangiopericytoma (3/0), Sarcoma (0/2), small cell carcinoma (1/0), basal cell carcinoma (0/1) | NA | 16 | 60 | 61.20 | 39 | NA | NA |
| Mortuaire G. | 2016 | RC | 2002–2013 | France | 43 | 20 | 23 | AC | 0 | 20 | 23 | 67.30 | 42 | NA | NA |
| Bhayani, M. K. | 2014 | RC | 1993–2009 | USA | 53 | 14 | 39 | AC | NA | NA | NA | NA | NA | NA | NA |
| Vergez, S. | 2012 | RC | 1999–2009 | France | 48 | 24 | 24 | AC | NA | 19 | 24 | 67.00 | 46 | 38.00 | 89.00 |
| Huber, G. F. | 2011 | RC | 1992–2007 | Switzerland | 18 | 12 | 6 | AC | 0 | 6 | 7 | 59.09 | 15 | 16.08 | 45.83 |
| Huang, Y. | 2018 | RC | 2001–2015 | China | 47 | 27 | 20 | NA | NA | NA | NA | NA | NA | 65.20 | 80.00 |
ACC, adenoid cystic carcinoma; AC, adenocarcinoma; CS, chondrosarcoma; DFS, disease-free survival; ER, endoscopic resection; FU, mean follow-up time; MECS, mesenchymal chondrosarcoma; MYCS, myxoid chondrosarcoma; NA, not available; NEC, neuroendocrine carcinoma; ON, esthesioneuroblastoma; OR, open resection; OS, overall survival; RC, retrospective cohort study; SCC, squamous cell carcinoma; SNMM, sinonasal melanoma; SNUC, sinonasal undifferentiated carcinoma.
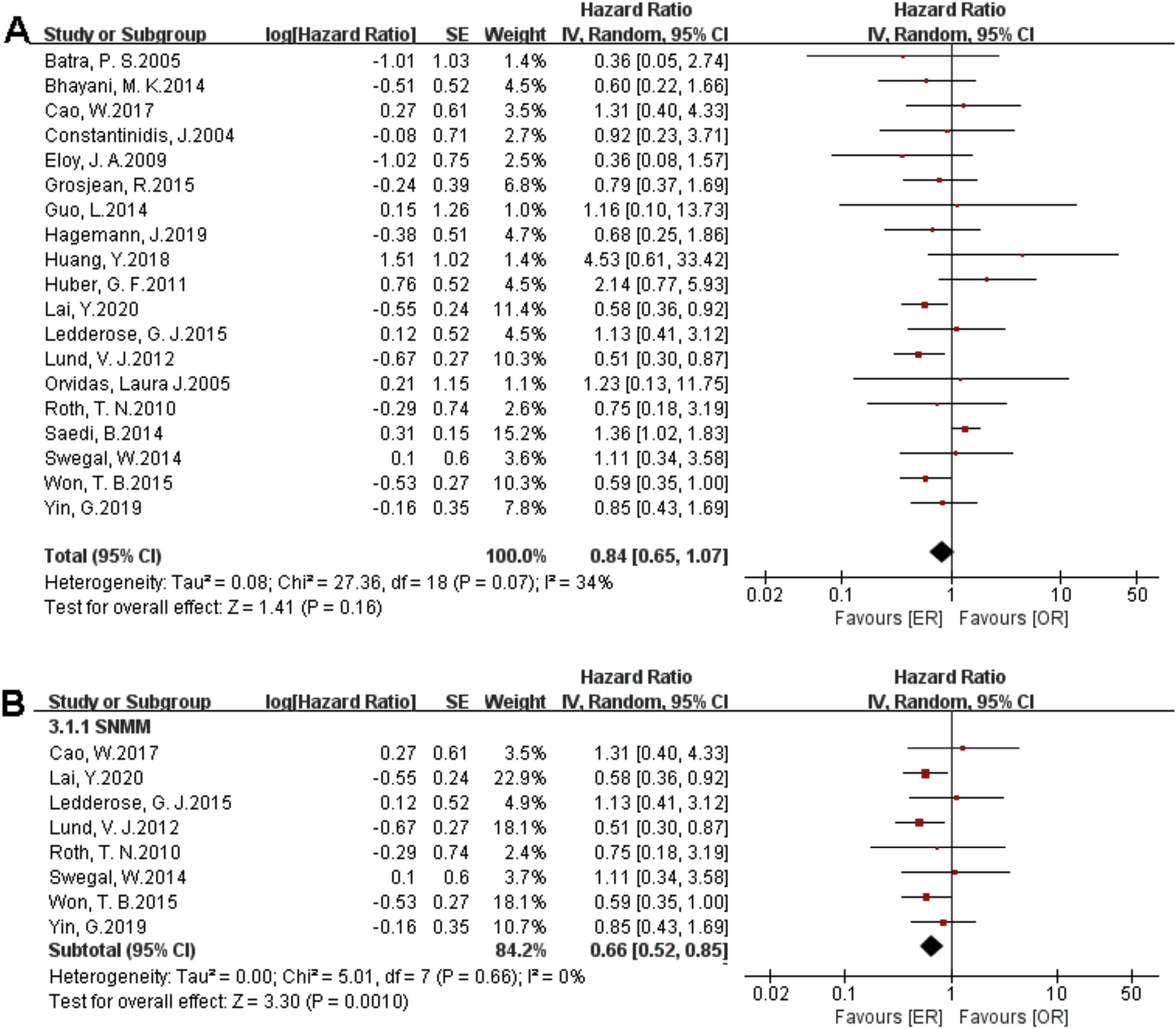
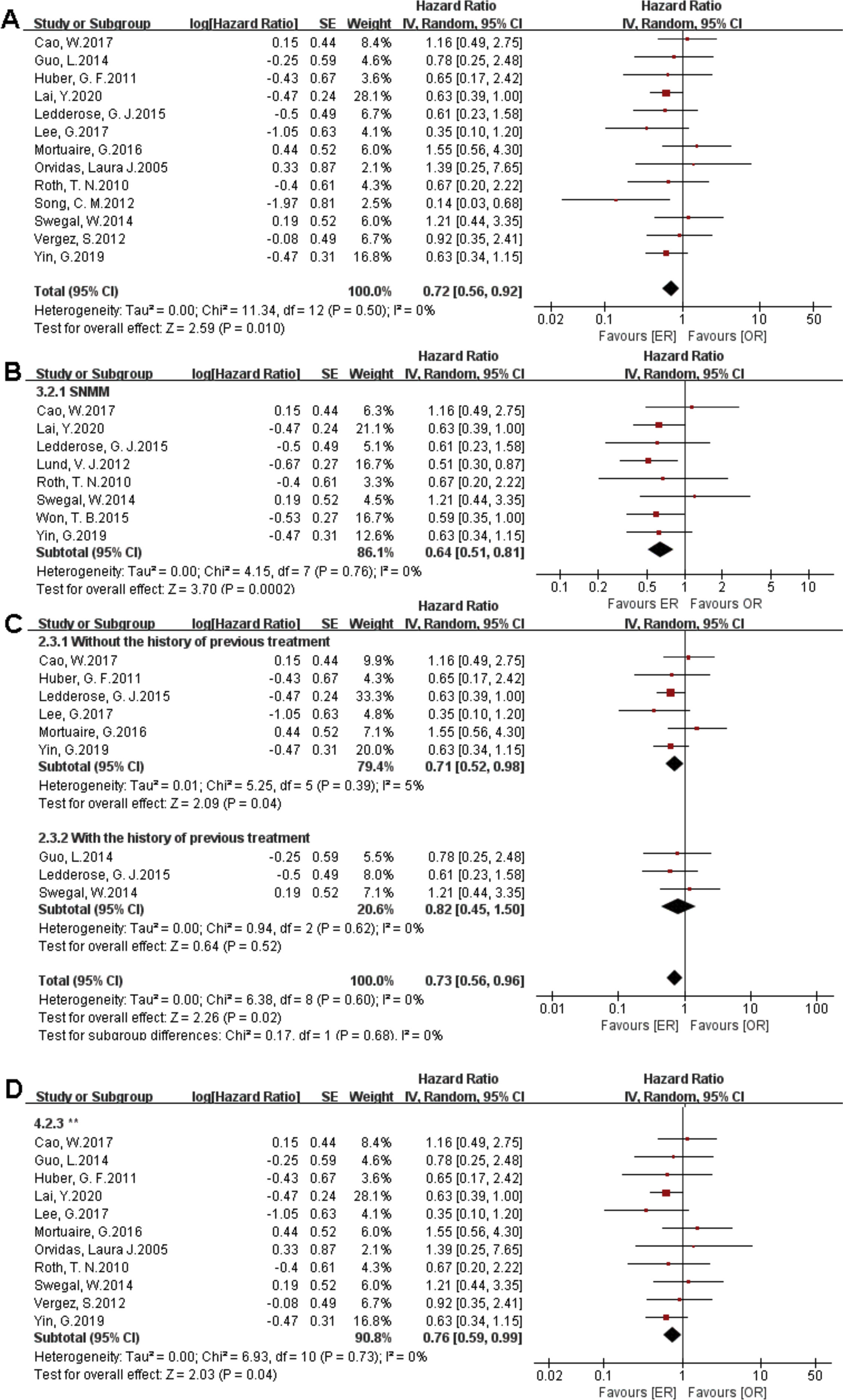
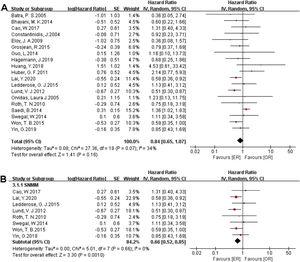
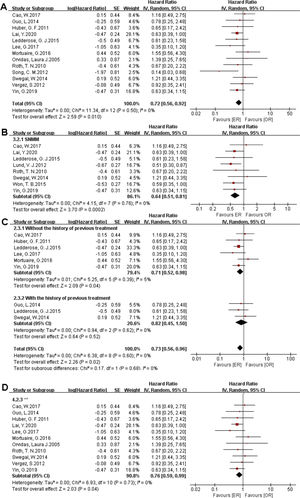
There were 1373 patients incorporated into our meta-analysis, of which 653 (47.56%) underwent surgery using the endoscopic approach, and 720 (52.44%) cases utilized open resection. Of the 23 articles included in the final meta-analysis, 19 studies (n = 1223 out of 1373) were included in the meta-analysis of OS. There was no significant difference in the OS between the endoscopic approach and the open approach (Fig. 2A), (HR = 0.84 [95% CI: 0.65–1.07], p = 0.16; random-effects analysis). Compared with the OR group, the OS rates in patients with sinonasal melanoma showed an advantage in the ER group (Fig. 2B), (HR = 0.66 [95% CI: 0.52–0.85], p = 0.001; random-effects analysis). Thirteen studies (n = 459 out of 1373) were included in the meta-analysis of DFS. The effect estimate suggested that the DFS of the ER group was higher than that of the OR group (Fig. 3A), (HR = 0.72 [95% CI: 0.56–0.92], p = 0.01; random-effects analysis). Compared with the OR group, the DFS rates in patients with sinonasal melanoma showed an advantage in the ER group (Fig. 3B) (HR = 0.64 [95% CI: 0.51–0.81], p = 0.0002; random-effects analysis). There was a significant difference in the DFS in cases without a previous treatment between the ER and OR groups (Fig. 3C), (HR = 0.71 [95% CI: 0.52–0.98], p = 0.04; random-effects analysis). The estimate effect of the HR of DFS favored the ER group in the subgroup with a higher comparability (Fig. 3D), (HR ≤ 0.76 [95% CI: 0.59–0.99], p = 0.04; random-effects analysis). There were no significant differences in the other subgroups (Supplementary Fig. 1).
Comparison between endoscopic resection and open resection of sinonasal malignancies disease-free survival in (A) all studies, (B) sinonasal melanoma subgroups, (C) with or without previous treatment subgroups and (D) disease-free survival of comparability subgroups. CI, confidence interval; ER, endoscopic resection; OR, open resection; SNMM, sinonasal mucosal melanoma.
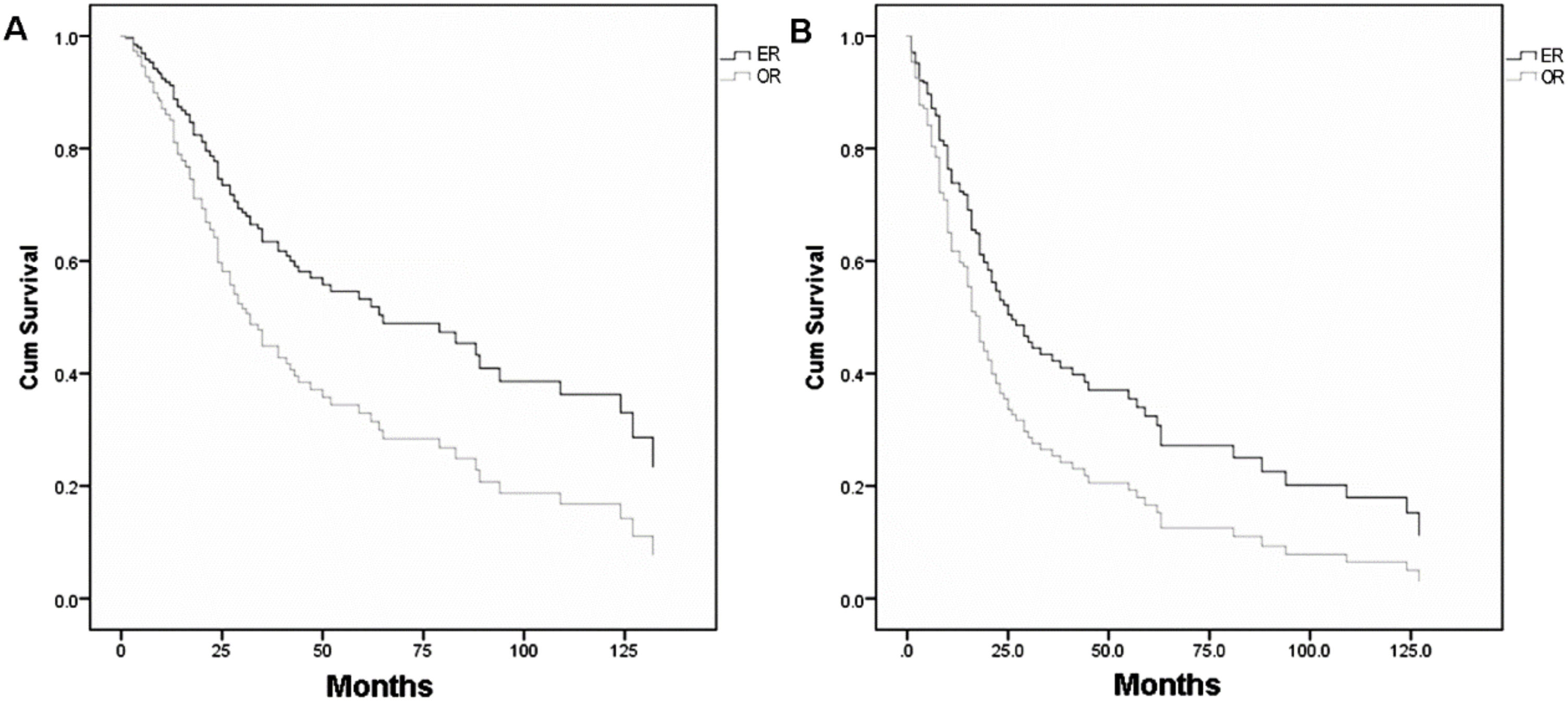
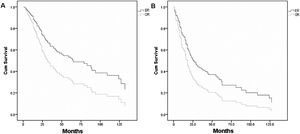
Table 213–15,17,18,23,35–38 shows the individual patient data derived from 10 articles which met the inclusion criteria of pooled-analysis. A total of 248 cases were included in the pooled-analysis and went through a direct comparison. In pooled-analysis, OS of the ER and OR group was 31.7% and 21.1% (p < 0.05), respectively. Table 3 indicates significant differences of OS in age, pathological type, T-stage, and adjuvant therapy with univariate analysis and in T-stage, adjuvant therapy, and surgical approaches (p = 0.006) (Fig. 4A) with multivariate analysis. DFS of the ER and OR group was 19.9% and 15.5% (p < 0.05), respectively. Table 4 indicates significant differences in DFS in age, pathological type, T-stage, and adjuvant therapy with univariate analysis and in adjuvant therapy and surgical approaches (p = 0.020) (Fig. 4B) with multivariate analysis.
Demographic data of pooled studies.
| Variable | ER | OR | X2 | p-Value |
|---|---|---|---|---|
| Age (mean ± SD) | 58.09 ± 17.58 | 58.12 ± 19.22 | 0.99 | |
| Histopathology | 16.25 | 0.002 | ||
| Adenocarcinoma | 15 (11.5%) | 26 (22.0%) | ||
| Chondrosarcoma | 8 (6.2%) | 13 (11.0%) | ||
| Melanoma | 70 (53.8%) | 41 (34.7%) | ||
| Esthesioneuroblastoma | 30 (23.1%) | 37 (31.4%) | ||
| SNUC | 7 (5.4%) | 1 (0.8%) | ||
| T stage | 0.66 | 0.42 | ||
| Low (T1–T2) | 25 (21.0%) | 14 (16.5%) | ||
| High (T3–T4) | 94 (79.0%) | 71 (83.5%) | ||
| Follow-up (median) | 25.2 | 35.2 | 0.09 | |
| Adjuvant therapy | 1.72 | 0.20 | ||
| No adjuvant therapy | 51 (41.8%) | 32 (30.5%) | ||
| Radiotherapy | 31 (25.4%) | 56 (53.3%) | ||
| Chemotherapy | 5 (4.1%) | 2 (1.9%) | ||
| Chemoradiotherapy | 35 (28.7%) | 15 (14.3%) | ||
| Total | 130 (52.4%) | 118 (47.6%) |
ER, endoscopic resection; OR, open resection; SD, standard deviation; SNUC, sinonasal undifferentiated carcinoma.
Cox proportional hazard analysis of overall survival.
| Variable | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Group (ER vs. OR) | 1.102 | 0.777–1.561 | 0.586 | 0.568 | 0.380–0.849 | 0.006 |
| Age | 1.034 | 1.023–1.046 | <0.001 | 1.003 | 0.988–1.018 | 0. 710 |
| Gender | 1.014 | 0.83–1.239 | 0.889 | |||
| Pathology | ||||||
| Esthesioneuroblastoma | Reference | <0.001 | 0.002 | |||
| Melanoma | 5.806 | 3.380–9.972 | <0.001 | 3.407 | 1.665–6.973 | 0.001 |
| SNUC | 2.541 | 0.735–8.785 | 0.141 | 1.374 | 0.358–5.272 | 0.644 |
| Adenocarcinoma | 1.761 | 0.870–3.567 | 0.116 | 1.310 | 0.416–4.129 | 0.645 |
| Chondrosarcoma | 0.472 | 0.137–1.625 | 0.234 | |||
| Stage (high vs. low) | 6.454 | 2.825–14.746 | <0.001 | 2.716 | 1.030–7.164 | 0.043 |
| Adjuvant therapy | 2.032 | 1.428–2.891 | <0.001 | 2.375 | 1.555–3.636 | <0.001 |
CI, confidence interval; ER, endoscopic resection; HR, hazard ratio; OR, open resection; SNUC, sinonasal undifferentiated carcinoma.
Cox proportional hazard analysis of disease-free survival.
| Variable | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Group (ER vs. OR) | 1.041 | 0.753–1.44 | 0.808 | 0.628 | 0.424–0.929 | 0.02 |
| Age | 1.026 | 1.016–1.037 | <0.001 | 1.008 | 0.993–1.024 | 0.275 |
| Gender | 0.983 | 0.679–1.424 | 0.928 | |||
| Pathology | ||||||
| Esthesioneuroblastoma | Reference | <0.001 | 0.004 | |||
| Melanoma | 6.224 | 3.433–11.282 | <0.001 | 4.225 | 1.934–9.232 | <0.001 |
| Adenocarcinoma | 2.533 | 1.271–5.049 | 0.008 | 3.117 | 1.228–7.911 | 0.017 |
| Chondrosarcoma | 2.109 | 0.953–4.665 | 0.066 | |||
| SNUC | 2.029 | 0.573–7.181 | 0.273 | 2.494 | 0.652–9.541 | 0.182 |
| Stage (High vs. Low) | 5.207 | 2.53–10.72 | <0.001 | 1.936 | 0.792–4.737 | 0.148 |
| Adjuvant therapy | 1.637 | 1.163–2.304 | 0.005 | 1.669 | 1.122–2.481 | 0.011 |
CI, confidence interval; ER, endoscopic resection; HR, hazard ratio; OR, open resection; SNUC, sinonasal undifferentiated carcinoma.
We conducted a meta-analysis of the available literature to compare the prognosis of sinonasal malignancies via endoscopic and open resection. Meanwhile a direct comparison was made between the groups from studies where the individual patient data was provided.
The meta-analysis indicated that the OS of the ER group was comparable with that of the OR group. This comparison of OS was, however, not stable. When we excluded Saedi’s study,24 the difference in the OS rates between the two groups turned into something meaningful (Supplementary Fig. 2), (HR = 0.72 [95% CI: 0.58–0.88], p = 0.002; random-effects analysis), which meant that the patients could benefit from ER in terms of OS rates. One explanation for the instability is that it arises from the relatively short follow-up time. The mean follow-up time of ER was 22 months, and that of OR was 20 months. However, the outcome of relapse requires a shorter follow-up time than death, which means OS needs longer follow-up time compared with DFS. In addition, the effect estimate suggested that DFS was higher when ER of sinonasal malignancies was performed.
The multivariate analysis of OS and DFS indicated a significant benefit of ER, which is different from univariate analysis. This variation may arise from the correlation between surgery approaches and the application of adjuvant therapy. There were 52.5% cases using adjuvant therapy in the endoscopic approach and 73.2% in the open approach (x2 = 7.559, p = 0.006). The multivariate analysis endorsed the application of adjuvant therapy as a protective factor. After eliminating the confounding factor through multivariate analysis, we found that surgery approaches have an independent effect on the survival outcomes. We are positive regarding the statistical result, considering the confidence interval of the effect estimate included appreciable benefit.
Rarity and heterogeneity of sinonasal malignancies contributed to the difficulty in the interpretation of survival results in the studies that reported different pathologies.5 Our multivariate analysis suggests that histopathology is an independent risk factor. A subgroup analysis was performed with studies where the pathological diagnosis was available. The effect estimate suggests that the outcome of the sinonasal melanoma in terms of OS and DFS is better for the endoscopic approach. In general, we believe that patients can benefit from ER. Since sinonasal melanoma is widely considered to be radioresistant, wide surgical excision is typically recommended as the primary mode of therapy.39,40 However, endoscopic resection may be able to provide a better outcome by enabling excellent vision that offers precise excision and better local control. The effect estimate in adenocarcinoma subgroup suggests a comparable outcome in terms of OS and DFS.
There was a statistical correlation between T stage and survival.41 Previous studies have reported ER as an alternative to OR in low stage sinonasal malignancies.5 The tumor stage relates to tumor invasion extent, which is one of considerations when designing surgical approach. The effect of tumor stage in survival between endoscopic and open resection cannot be meta-analyzed as the sequence of the incompleteness of data, as well as the tumor invading site.
Adjuvant therapy plays a role in increasing the cure rate of sinonasal malignancies. Our multivariate analyses indicated that the adjuvant therapy was a protective factor for OS and DFS. Although the data in the literature provided were inadequate to conduct a subgroup analysis of the adjuvant therapy, the relationship between adoption of adjuvant therapy and selection of surgical approaches should not be underestimated. It is of much concern to develop a multidisciplinary therapy.
The advantages of endoscopic approach are technically clear. An endoscopic approach would be advocated for pathologies that surgical excision is recommended as primary therapy, based on the data summarized above. Meanwhile, endoscopic approach with or without auxiliary incision showed significant benefits for skull base involvement. But when an ocular enucleation or a total maxillectomy is required according to the extent of tumor, leading to inevitable facial deformity, an open surgical approach could benefit the patient. Lesions involving vital structures such as internal carotid artery are generally excised by the open approach according to the conventional viewpoint, but the endoscopic approach is an alternative due to the development of minimally invasive surgery technology and the improvement of surgical technique proficiency.
Of the 23 studies evaluated using NOS, 6 had 6 stars, 14 had 7 stars, and 3 had 8 stars (Table 5), whereas the maximum possible total score for a cohort study is 9 stars. The levels of evidence were accessed by the GRADEpro system. The certainties of effect estimate of OS and DFS were very low, on account of the imprecision and publication bias (Table 6). Moreover, the downgrading was on account of the following two aspects: 1) The confidence interval of the effect estimate contained an invalid value and included appreciable benefit42; and 2) The studies included in the analysis were observational studies. As a result, we were unable to ascertain whether the studies could represent all cases.43 However, due to the rarity of sinonasal malignancies, it would be difficult to plan a prospective randomized cohort study.
Quality assessment of included studies by Newcastle-Ottawa assessment scale (NOS).
| Study | Year | Nº of stars | |||
|---|---|---|---|---|---|
| Selection | Comparability | Outcome | Total | ||
| Constantinidis, J. | 2004 | 3 | 1 | 2 | 6 |
| Orvidas, Laura J. | 2005 | 3 | 2 | 2 | 7 |
| Roth, T. N. | 2010 | 3 | 2 | 2 | 7 |
| Lund, V. J. | 2012 | 3 | 1 | 3 | 7 |
| Song, C. M. | 2012 | 3 | 1 | 2 | 6 |
| Guo, L. | 2014 | 3 | 2 | 3 | 8 |
| Saedi, B. | 2014 | 3 | 2 | 2 | 7 |
| Swegal, W. | 2014 | 3 | 2 | 2 | 7 |
| Grosjean, R. | 2015 | 3 | 1 | 2 | 6 |
| Ledderose, G. J. | 2015 | 3 | 1 | 2 | 6 |
| Won, T. B. | 2015 | 3 | 1 | 2 | 6 |
| Cao, W. | 2017 | 3 | 2 | 2 | 7 |
| Hagemann, J. | 2019 | 3 | 2 | 2 | 7 |
| Yin, G. | 2019 | 3 | 2 | 2 | 7 |
| Lai, Y. | 2020 | 3 | 2 | 2 | 7 |
| Lee, G. | 2017 | 3 | 2 | 2 | 7 |
| Batra, P. S. | 2005 | 3 | 2 | 2 | 7 |
| Eloy, J. A. | 2009 | 3 | 2 | 2 | 7 |
| Mortuaire, G. | 2016 | 3 | 2 | 2 | 7 |
| Bhayani, M. K. | 2014 | 3 | 1 | 2 | 6 |
| Vergez, S. | 2012 | 3 | 2 | 3 | 8 |
| Huber, G. F. | 2011 | 3 | 2 | 3 | 8 |
| Huang, Y. | 2018 | 3 | 2 | 2 | 7 |
Outcomes assessment of included studies by GRADE.
| Outcome | Certainty assessment | HR (95% CI) | Certainty | Importance | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nº of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | ||||
| OS | 19 | Observational studies | Not serious | Not serious | Not serious | Seriousa | Publication bias strongly suspectedb | 0.84 (0.65–1.07) | ⨁◯◯◯ | Critical |
| Very low | ||||||||||
| DFS | 13 | Observational studies | Not serious | Not serious | Not serious | Seriousa | Publication bias strongly suspectedb | 0.84 (0.61–1.14) | ⨁◯◯◯ | Critical |
| Very low | ||||||||||
CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; OS, overall survival.
There are some limitations of our study. First, the low quality of evidence is almost inevitable for observational studies, although the existence of some relevant factors could make it possible to improve the quality of the evidence, for example increasing the sample size to avoid imprecision. Second, the effects of adjuvant therapy, previous treatment, and histopathology were not analyzed adequately. Although subgroup analyses were planned to be conducted, the data reported by most of studies were deficient to perform such an analysis. Hence, further exploring the standardization of the reports would make sense.5,6 At last, to the best of our knowledge, our study is the first one conducting a meta-analysis of the direct comparison between ER and OR groups. However, the effect estimate was not sufficiently stable. A longer follow-up time and more standard management are essential to improve the statistical power for further analysis.
ConclusionThe evidence we collected suggests that the survival outcome of endoscopic resection in patients with sinonasal malignancies was comparable or better than that of open resection. The factors associated with tumor prognosis are histopathology, stage of tumor, and application of adjuvant therapy. Further research will be important to establish the guidelines for the selection of surgical approach and promote the comprehensive treatment of sinonasal malignancies.
Conflicts of interestThe authors declare no conflicts of interest.
Data collection: Sijie Jiang and Ruohao Fan.
Data analysis and draft writing: Sijie Jiang.
Technical and material support: Weihong Jiang and Ruohao Fan.
Study design and supervision: Zhihai Xie and Hua Zhang.
This study was supported by the Fundamental Research Funds for the Central Universities of Central South University (No.2020zzts864) and National Natural Science Foundation of China (No. 81770985 and No. 81873695).