Behavioral and electrophysiological auditory evaluations contribute to the understanding of the auditory system and of the process of intervention.
ObjectiveTo study P300 in subjects with severe or profound sensorineural hearing loss.
MethodsThis was a descriptive cross-sectional prospective study. It included 29 individuals of both genders with severe or profound sensorineural hearing loss without other type of disorders, aged 11 to 42 years; all were assessed by behavioral audiological evaluation and auditory evoked potentials.
ResultsA recording of the P3 wave was obtained in 17 individuals, with a mean latency of 326.97ms and mean amplitude of 3.76V. There were significant differences in latency in relation to age and in amplitude according to degree of hearing loss. There was a statistically significant association of the P300 results with the degrees of hearing loss (p=0.04), with the predominant auditory communication channels (p<0.0001), and with time of hearing loss.
ConclusionsP300 can be recorded in individuals with severe and profound congenital sensorineural hearing loss; it may contribute to the understanding of cortical development and is a good predictor of the early intervention outcome.
As avaliações comportamentais e eletrofisiológicas auditivas contribuem para o entendimento do sistema auditivo e do processo de intervenção.
ObjetivoEstudar P300 em indivíduos com perda auditiva sensorioneural severa ou profunda.
MétodoEstudo prospectivo transversal descritivo. Participaram 29 indivíduos, de ambos os sexos, com idade entre 18 e 45 anos com perda auditiva sensorioneural, congênita severa ou profunda e sem comorbidades, avaliados por meio de avaliação audiológica comportamental e potencial evocado auditivo de longa latência.
Resultadoso registro da onda P3 foi obtido em 17 indivíduos, com latência e amplitude média de 326,97ms e 3,76V, respectivamente. Houve diferenças significativas da medida de latência em relação à idade e da amplitude segundo o grau da perda auditiva. Evidenciou-se associação do resultado do P300 aos graus de perda auditiva (p=0,04) e ao canal de comunicação auditiva predominante (p=0,0001) e ao tempo de privação auditiva (teste exato de Fisher).
ConclusõesP300 pode ser registrado em indivíduos com perda auditiva sensorioneural congênita e colaborar para a compreensão do desenvolvimento cortical auditivo e ser preditor do resultado da intervenção.
There is a long history of investigation of the function of the auditory system, the disorders that affect it and intervention strategies to mitigate the ill effects of those disorders.
Increasingly, investigators have studied the peripheral and central auditory system using objective and non-invasive techniques, such as behavioral tests that assess auditory processing, as well as electrophysiological evaluation, principally auditory evoked potentials (AEPs).1–10
The auditory event-related evoked potential (P300) provides an objective measure of central auditory function, as it reflects the cortical electrophysiological activity involved in attention, discrimination, memory, integration, and decision-making skills.11
The few reports in the literature that have recorded cortical auditory event-related evoked potentials (P300) in individuals with hearing loss frequently consist of only a small number of individuals, and these studies have reported divergent results.
Peripheral hearing loss can indirectly affect recordings of the latency of the P3 and the N1–P2–N2 complex. Another factor that impacts the results of P300 recordings is a difference in hearing thresholds at two frequencies, commonly observed in the elderly and in hearing loss with a descending pattern.12
However, a peripheral hearing loss does not invalidate the use of this measure, as long as the individual is capable of perceiving the stimulus.13
Latency measures are known to be sensitive indicators in individuals with hearing loss and the degree of hearing loss can affect the amplitudes of the components of auditory evoked potentials (AEPs) in different ways.
Age can also influence latency measures, as can other factors. Values close to 350ms are considered normal for the P300 latency in adults younger than 45 years of age; after that age, it has been proposed to add 10ms for each decade of life.14–16
The aim of this study was to investigate the auditory evoked potential P300 in individuals with severe to profound congenital hearing loss and to correlate the results with age, gender, degree of hearing loss, time of auditory deprivation, and the predominant communication modality (auditory or visual).
MethodsThis was a descriptive, contemporary, cross-sectional cohort study. The study was approved by the research ethics committee of a public university in the state of São Paulo – SP (protocol #1011/01). All participants or guardians received an invitation letter with information about the study and signed the informed consent.
A total of 29 individuals (15 males and 14 females), between the ages of 18 and 45 years, participated in the study. The inclusion criteria were: adult subjects of both genders, aged 18–45 years, with, bilateral, symmetric, prelingual, severe-to-profound sensorineural hearing loss, exhibiting hearing thresholds between 70 and 90dBHL in at least two frequencies in both ears and with no other disorders or evidence of central auditory hearing impairment.
Initially, a review of the medical records and a structured interview were performed to obtain personal data related to the subject's history and type of rehabilitation. Behavioral, audiological, and impedance assessments were performed to ensure the subjects’ eligibility.
Electrophysiological assessment was performed using the long-latency auditory evoked potential (P300) recording. Bio-logic Systems Corp. equipment was used for the P300 recording. The active electrodes were placed on the forehead (Fpz=ground electrode), the cranial vertex (Cz=active electrode), and the earlobes (reference electrode: A1=LE and A2=RE), according to the International 10-20 System,17 and headphones were used (TDH-39).
For the electrophysiological assessment, we required that each electrode have an impedance ≤3kΩ and the impedance between electrode pairs was <3kΩ. The examination was performed with the volunteer in supine position, in a quiet environment. The volunteer was instructed to remain as quiet as possible, with eyes directed at a specific point in the room, and to pay attention to different stimuli that occurred infrequently and randomly among a series of similar and much more frequent stimuli. At this stage of the examination, we verified that the participants understood the nature of the test in order to avoid degrading the results. For individuals who used Brazilian Sign Language (Língua Brasileira de Sinais [LIBRAS]) and required an interpreter, the test was scheduled on a specific date to ensure the presence of the interpreter and comprehension of the test by the participant.
All participants had undergone childhood phonoaudiological rehabilitation programs and the fitting of hearing aid amplification bilaterally, during the process of intervention with oral method. The subjects who used LIBRAS acquired this language skill in adulthood.
Each participant was instructed regarding care related to variables that could have an impact on cognitive potential results,18–21 such as avoiding medications 24h before the test, and, in the four hours prior to the examination, avoiding strenuous physical or mental activity, smoking or the use of stimulants such as tea, coffee, or chocolate, and the use of facial cream or hair gel.
Each participant was asked to respond to the test by slightly raising the index finger as the appropriate response to the detection of the uncommon stimulus each time it appeared.
Before initiating the recording, the subject was given a training session of stimuli, and counseled that the uncommon stimuli could take time to appear, or could appear after only a very brief time interval. After the exposure and training time to ensure the subject's understanding, the test was initiated and the recording was made.
The stimulus intensity for the elicitation of P300 ranged from 20 to 25dBSL (decibel sensation level, i.e., 20–25dBSL above the auditory threshold for the frequency used) for the frequencies used in the frequent and uncommon stimuli, to facilitate detection of auditory stimuli by the study participant. If this level of stimulus presentation caused discomfort, the highest level of comfort reported by the patient at which he or she could detect the sound was used.
The following parameters were used for the acquisition of P300: binaural acoustic stimuli (tone bursts with 50ms duration, with plateau of 30ms and a rise/fall time of 10ms) of low frequency, present in the assessed subject for the frequent stimulus (probability of 80%) and a higher, uncommon stimulus (probability of 20%). The frequency and intensity of both the frequent and the uncommon stimuli were selected by the pure tone audiometry, i.e., frequencies with detectable thresholds. The stimulus intensity also varied according to the frequency used and the hearing threshold, making sure that the stimuli were always suprathreshold.
Three-hundred artifact-free stimuli (approximately 60 uncommon and 240 frequent stimuli) were used to obtain the potentials. The firing frequency or rate of presentation was one stimulus per second.
The N1, N2, and P2 complex was not analyzed, as the physical characteristics of the stimulus were adjusted to individual needs as described above. After ensuring the detection of stimuli by the assessed individuals, the N1, P2, and N2 complex appeared in the 29 studied subjects.
The markings of the tracing used to obtain the amplitude and latency of P300 were performed by three professionals with experience in electrophysiology. When the tracing was considered difficult to analyze, i.e., there was no agreement concerning the marking, it was discussed by the professionals until a consensus was attained.
The statistics used in the comparison of the groups formed during the course of the present study followed the literature guidelines22,23 and the significance level was set at 5% (p<0.05). Significant results are shown in bold.
ResultsWe were successful in recording the P300 component in 58.6% of the subjects studied (n=29).
The comparison of the amplitude and latency values of the P300 component, with appropriate statistical description for each study group considering gender, is shown in Table 1. It can be observed that there was no statistically significant difference between genders.
Descriptive analysis of amplitude (μV) and latency (ms) of P300 according to gender.
| Latency (ms) | Amplitude (μV) | |||||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||||
| CzA2 | CzA1 | CzA2 | CzA1 | CzA2 | CzA1 | CzA2 | CzA1 | |
| Mean | 331.9 | 325.4 | 322.9 | 327.3 | 4.45 | 4.35 | 2.77 | 3.29 |
| Median | 341.0 | 324.0 | 321.0 | 319.0 | 3.18 | 3.22 | 2.60 | 2.93 |
| SD | 45.85 | 42.96 | 41.14 | 34.61 | 3.14 | 2.34 | 1.40 | 1.26 |
| SE | 15.28 | 14.32 | 14.54 | 12.24 | 1.05 | 0.78 | 0.50 | 0.45 |
| p (paired Student's t-test) | 0.45 | 0.61 | 0.88 | 0.17 | ||||
Significant differences in latency measurements were observed at the positions of electrodes CzA2 and CzA1 between the different age groups (Table 2). A significant difference was observed in amplitude for the degree of hearing loss, as shown in Table 3.
Amplitude values (μV) and latency (ms) for the P300 component considering two groups according to the age range (11–24 years and 25–45 years).
| Amplitude(μV) | Latency (ms) | |||||||
|---|---|---|---|---|---|---|---|---|
| Electrode CzA2 | Electrode CzA1 | Electrode CzA2 | Electrode CzA1 | |||||
| G 11–24 | G 25–45 | G 11–24 | G 25–45 | G 11–24 | G 25–45 | G 11–24 | G 25–45 | |
| Mean | 3.70 | 3.46 | 3.80 | 4.06 | 318.3 | 371.3 | 318.1 | 364.3 |
| Median | 2.80 | 2.75 | 3.03 | 4.17 | 316.0 | 372.0 | 312.0 | 357.0 |
| SD | 2.79 | 1.30 | 2.09 | 1.17 | 40.97 | 11.02 | 36.18 | 20.98 |
| SE | 0.74 | 0.75 | 0.56 | 0.67 | 10.95 | 6.36 | 9.67 | 12.12 |
| p (Mann–Whitney) | 0.33 | 0.33 | 0.03 | 0.02 | ||||
Descriptive measures of latencies (ms) and amplitude (μV) of P300, according to the variable degree of hearing loss.
| Amplitude(μV) | Latency (ms) | |||
|---|---|---|---|---|
| Profound | Severe | Profound | Severe | |
| Mean | 2.69 | 5.98 | 331.0 | 318.5 |
| Median | 2.80 | 5.24 | 322.0 | 322.0 |
| SD | 1.00 | 2.49 | 39.75 | 40.35 |
| SE | 0.21 | 0.75 | 8.29 | 12.17 |
| p (Mann–Whitney) | 0.0015 | 0.40 | ||
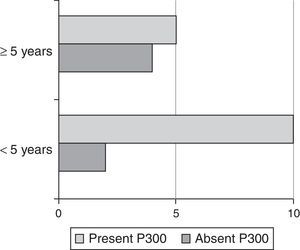
Fisher's exact test showed an association between the presence or absence of P300 with time of auditory deprivation (Fig. 1) and the periods of auditory stimulation initiation, i.e., the critical periods for auditory perception development, when comparing a group with intervention before 5 years of age and the group that started the intervention after this age.
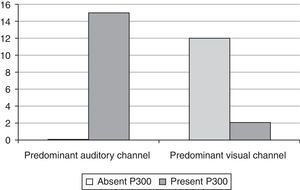
The chi-squared test showed an association of the result to the predominant auditory communication modality with the presence of P3 (p<0.0001) (Fig. 2).
DiscussionNo statistically significant differences were observed for the amplitude measurements related to gender and age; nor for differences in latency related to gender (Tables 1 and 2). When latency measurements were compared in two study groups of different ages (Table 2), statistically significant differences were observed, longer latencies in the age group of 25–45 years than in the group of 11–24 years, consistent with other reports in the literature.9
Many studies have reported differences in the P300 latency and amplitude among different age ranges.14–16 These studies suggest that maturation is reflected in a variation of the amplitude and latency of P1, N1, and P2 components, with an increase in the amplitude and a decrease in latency in younger children, that changes with maturation. Despite the increasing number of studies on variables such as amplitude, latency, and age, normative data on the P300 response in children are still scarce, especially in younger children and infants. For these populations, studies are being conducted with the recording of P3a (passive P300 response)15 and the latency of P1, considered as a biomarker for central auditory development in children with hearing impairment.8
For school-age children from 6 years of age to late adolescence, there are more studies using conventional P300. In this age range, latency decreases and, amplitude increases and the morphology of P300 becomes more well defined.14,24,25 There is a significant correlation between age and maturation of the auditory system, with approximately a 20ms/year reduction in latency occurring up to age 15 years,26,27 and an increase in latency with further aging, a finding also observed in the studies of Frizzo and Junqueira for the age group of 8–36 years.28
The effect of aging on P300 response is probably the most extensively studied variable in the past decades.16,29,30 There is no consensus in the literature regarding the fact that aging clearly affects measures of latency and amplitude of P300, but it must be remembered that there is also great variability, both intrinsic (gender, intellectual level, task type, etc.) and extrinsic (stimulus parameters and potential capture method, etc.). among the studies and these will bear upon on the specific conclusions for different age groups in the various studies.
Studies have suggested that there is an increase in latency of approximately 1–2ms/year, a decrease in amplitude in accordance with a mean of 0.2μV/year, and also a possible association between age and scalp topography in the measurement of P300.31,32 The best recording of age-related changes in latency and amplitude of P300 occurs when electrodes are placed in the region of the central and parietal gyri (Cz and Pz), rather than when the forehead (Fz) and a lateral electrode are used.32
It should be emphasized that with increasing patient age, professionals should be more careful when recording P300, as both thresholds and the index of speech recognition are factors that commonly change with aging and, can be altered by impairment of either the auditory periphery or the central auditory pathways. Auditory sensitivity may be a determinant factor in reducing the occurrence of the P300 response with age.16 Thus, these variables must be controlled in clinical protocols of evoked potentials when evaluating elderly and younger adults, and should include at least pure tone audiometry and speech audiometry in the tests.
In contrast to age, gender did not appear to be a significant variable in the measurements of latency and amplitude of P300.16,33 However, there are reports of greater amplitude of the P3 wave and lower latency for women over 15 years of age.14,19
Regarding the degree of hearing loss, no significant difference was observed in mean latency between the two groups (severe and profound hearing loss). P300 values were similar in both groups (Table 3), suggesting that peripheral hearing loss did not invalidate the use of this measure, as previously theorized in the literature.1 P300 cannot be recorded if the thresholds for both the uncommon and the frequent stimuli are below the audibility threshold.34 P300 can be correlated with the degree of overall cognitive impairment rather than with any specific diagnosis, as the response is altered by a wide variety of disorders that affect cognition.35
One of the proposed uses of the P300 recording is to monitor the effects of an intervention, documented by a reduction in latency with increased cognitive capacity.36,37 For this, P300 is useful because of one of its more stable parameters, i.e., intra-subject measurement. Some studies have questioned the effect of peripheral hearing loss on the several components of the cortical auditory evoked potential associated with auditory behavioral assessment, as the effects of hearing loss on speech perception capacity, determined by auditory behavioral assessment, are reasonably well known.6
When we compared amplitudes in groups of individuals with severe and profound hearing loss (Table 3), we noted a significant difference in the mean amplitude between the two groups (p=0.0015). Studies have shown that when sensorineural hearing loss increases, there is a significant reduction in amplitude and a prolongation of latency for all components of the AEP.6
We found a significant association between the presence of P300 and the earlier the age at which subjects began the rehabilitation process (Fig. 1), consistent with the literature findings.6,8,38–40
A statistically significant P300 recording (p<0.0001) was also related to the predominant auditory communication modality (Fig. 2). These results raise many intriguing questions that have potential implications for effective adjustments in amplification and development of rehabilitation strategies for individuals with sensorineural losses that still need to be investigated.
In subjects who can hear, functional magnetic resonance imaging demonstrates that areas of sound activation are represented by a complex interactive network including regions of the posterior parietal cortex, dorsolateral prefrontal cortex, and inferior frontal cortex.
Temporal analysis suggests that the spatial discrimination of sound begins in a left to right direction in regions that are adjacent to the primary auditory cortex (superior temporal gyrus), whereas hemispatial integration and that of the eccentric areas may occur later. Activations have been identified in dorsal and ventral auditory pathways, which are presumed to be preferentially related to spatial and non-spatial analysis of sound, respectively. Activation findings in the ventral pathways could otherwise reflect the well-known functional duality of spectral analysis, that is, the concurrent extraction of information based on location due to the spectrotemporal distortions caused by the head and auricle, as well as the spectral characteristics of the sound source.41,42
Considering brain functions related to sign language, the present study observed greater activation in the left superior temporal sulcus and gyrus, extending further to areas of the supra-marginal gyrus in sign language readers (both hearing and non-hearing) than in non-readers (hearing). This would suggest an important role of the planum temporale in any mode of communication, since it responds to visual motion that occurs in the perception of gestures, both in deaf individuals and those who can hear. It is important to remember that in congenital deafness, the role of visual processing is more important.43
During the assessment of the meaning of the signs of hand gestures, the inferior parietal, superior temporal, and inferior occipito-temporal sulcus regions were simultaneously activated with the same time course of electrical activity as measured by magnetoencephalography, suggesting integration between the dorsal and ventral pathways of the superior temporal sulcus. Another finding was the marked predominance of right hemisphere participation, suggesting that processing of manual expression is similar to that of social cues, such as facial expression.44
Considering the extent of uptake and processing of stimuli at a more posterior cortex level in subjects using visual communication (compared to those who could hear), our failure to detect the P300 in subjects with a visual communication modality raises the question of the location of the electrode on the scalp, which seems to deserve further investigation. The P300 amplitude changes according to the placement of electrodes in the midline, typically increasing from the frontal toward the parietal regions.45 Thus, in subjects using visual communication, P300 could have higher amplitude if the electrode were placed on Pz rather than Cz, as typically used.
The predominance of electrical activation in the right hemisphere44 suggests the inversion of the left/right direction,41 and if that occurs, there are consequences for the tracing of the evoked potential on the scalp.
It is noteworthy that the process generating the P300 is modulated by the level of attention available at the time of the testing, and more difficult tasks requiring more attention, tend to reduce the amplitude and increase latency.46 In this sense, it can be speculated that in patients using manual communication, when making a greater effort to detect the uncommon tone, even if the uncommon tone is detected, the potential is not produced at sufficient amplitude to be measured. Other factors that could reduce the amplitude of the P300 include: the stimulus was heard for the first time,47 without prior training, which did not occur in the subjects of this research; parietal-temporal integrity failure,48 which was not assessed here, and a short interval between the target or uncommon stimuli,49 considering that in this study both subjects with training by auditory pathway as well as visual pathway had the same test pattern.
ConclusionsThis study concluded that the P300 can be recorded in individuals with severe and profound congenital sensorineural hearing loss.
Measurements of P300 did not show any differences when compared for age and gender. However, there are differences in the degree of hearing loss (severe and profound) and there is an association between the absent and present P300 and the subject's age at the time of the start of the (re)habilitation process, as well as the predominant communication modality (auditory-present) of the assessed subject.
Due to the non-detection of P300 in subjects who predominantly use a visual communication modality, the authors emphasize the importance of research in auditory and visual functions of these individuals to verify the association between communication deficits and the possibilities of intervention required in this population.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Reis AC, Frizzo AC, Isaac ML, Garcia CF, Funayama CA, Iório MC. P300 in individuals with sensorineural hearing loss. Braz J Otorhinolaryngol. 2015;81:126–32.