Pharyngocutaneous fistula is considered one of the major complications in the post-operative period after total laryngectomy/pharyngolaryngectomy, leading to a severe adverse impact for the patient and society. This study aimed to identify all the described pharyngocu-taneous fistula predictive factors and risk classifications.
MethodsResearch was conducted to identify all the studies assessing predictive factors and risk classification for pharyngocutaneous fistula development published until April of 2012 (n=846). The included studies were analyzed and data regarding their identification, methodological quality and results were recorded.
ResultsA total of 39 studies were included. The variables consistently reported as associated with fistula development were nutritional deficiency, American Society of Anesthesiologists (ASA) classification, high consumption of alcohol, anemia and hypoalbuminemia, co-morbidities, advanced N stage, location and extent of primary tumor, pre-radiotherapy and pre-chemo- radiotherapy treatment, emergency tracheotomy, surgical margin status, surgery’s duration, surgeon’s experience, local complications of the wound, performance of intraoperative blood transfusion and relationship between nasogastric tube and oral feeding.
ConclusionSeveral risk factors were associated with pharyngocutaneous fistula formation in the included studies. However, there is still no consensus in the most pertinent selection. Only two classification systems were retrieved and they were not able to accurately predict pharyngocutaneous fistula.
© 2014 Associação Brasileira de Otorrinolaringologia e Cirurgia Cérvico-Facial. Published by Elsevier Editora Ltda. All rights reserved.
Fístula faringocutânea éuma das maiores complicações pós-operatórias de laringectomia total e faringolaringectomia, podendo causar graves efeitos adversos ao paciente e à sociedade. Nosso objetivo é identificar todos os fatores preditivos e as classificações do risco descrito para a formação da fístula.
MétodoPesquisa racional foi realizada para identificação de todos os estudos sobre fatores preditivos e classificações/probabilidade de risco ao desenvolvimiento da fistula faringocutânea, publicados até abril de 2012 (n=846). Os estudos incluídos foram analisados e os dados, a identificação, a qualidade metodológica e os resultados foram registrados.
ResultadosUm total de 39 estudos foi incluído. As variáveis relacionadas foram deficiência nutricional,ASA,alto consumo de àlcool, anemia e hipoalbuminemia,comorbilidades,Navancado,localização/extensão do tumor primário,tratamentospré-cirúrgicos radioterapia/ quimioradioterapia, traqueostomia de emergência, status das margens cirúrgicas, duração da cirurgia, experiência do cirurgião, complicações locais da ferida cirúrgica, transfusão de sangue intraoperatória e relação entre sonda nasogástrica e alimentação oral.
ConclusãoNos estudos incluídos, muitos fatores de risco são associados ao desenvolvimento da fístula faringocutânea. Entretanto, ainda não existe um consenso sobre a seleção dos fatores mais influentes. Foram encontrados somente duas classificações de riscos e nenhuma delas foi preditiva para o do desenvolvimento da fístula faringocutânea.
Pharyngocutaneous fistula (PCF) can be defined as a dehiscence of the closure of the pharyngeal mucosa, resulting in leakage of saliva and communication with the skin.1 It is considered as one of the major complications in the postoperative period after total laryngectomy and pharyngo- laryngectomy, leading to a severe adverse impact for the patient and society.
The reported incidence ranges from 5% to 65% in the years 70th and 80th and between 9% to 25% in the last decade. Its occurrence vastly increases the length of stay and consequently, the costs of treatment.2 Additionally, this complication can lead to a delayed onset of complementary therapies (such as radiotherapy/chemotherapy), which in turn increases the physical and mental weakness of the patient due to the delay of oral feeding onset and voice rehabilitation, thus hampering the postoperative recovery. In rare cases, it can also lead to stenosis and pharyngeal swallowing disorders, with large negative impact on quality of life for patients,1–3 or even salivary dissection, rupture of the carotid artery, sepsis, mediastinitis, pneumonia and death.
Many factors are described as predisposing to fistula formation; they can be divided as patient-, disease-, and treatment-related factors.4,5 However, there is still no consensus among authors regarding the most significant risk factors for the occurrence of this complication.5,6
Therefore, this systematic review was conducted in order to determine the predictive variables associated with pharyngocutaneous fistula development and the existing risk classifications.
Data sources and review methodsA systematic review was performed in order to identify all the studies published through April of 2012, assessing variables and risk classifications for pharyngocutaneous fistula occurrence prediction in accordance with the methodology described in the Cochrane Handbook for Systematic Reviews of Interventions, 2008.7
The research sources used were: MEDLINE, Scopus, Cochrane Central, Cochrane Database of Systematic Reviews and clinicaltrials.gov, as well as reference lists from selected articles and pertinent systematic reviews.
The survey was conducted through MeSH terms (postoperative complications, fistula, pharyngocutaneous fistula, cutaneous fistula, risk factors, laryngectomy and pharyn- golaryngectomy) and their combination through Boolean operators. The inclusion criteria were: a) primary site of the tumor: laryngeal and/or hypopharynx, subdivided into supraglottic, glottic, infraglottic and hypopharynx that included primary neoplasm of pyriform sinus; b) type of surgery performed: total laryngectomy, total laryngectomy with partial pharyngectomy with or without neck dissection, salvage surgery for primary tumor of the larynx and hypopharynx; c) reconstruction: surgery with primary pharyngeal closure; and d) studies evaluating the association of risk factors with pharyngocutaneous fistula.
The exclusion criteria were: a) studies of opinion, case series and reviews; b) studies with outcome of general complications related to the intervention without the individual analysis of outcome data for the fistula formation; and c) studies written in foreign languages without adequate translation into English, Spanish, French, or Portuguese.
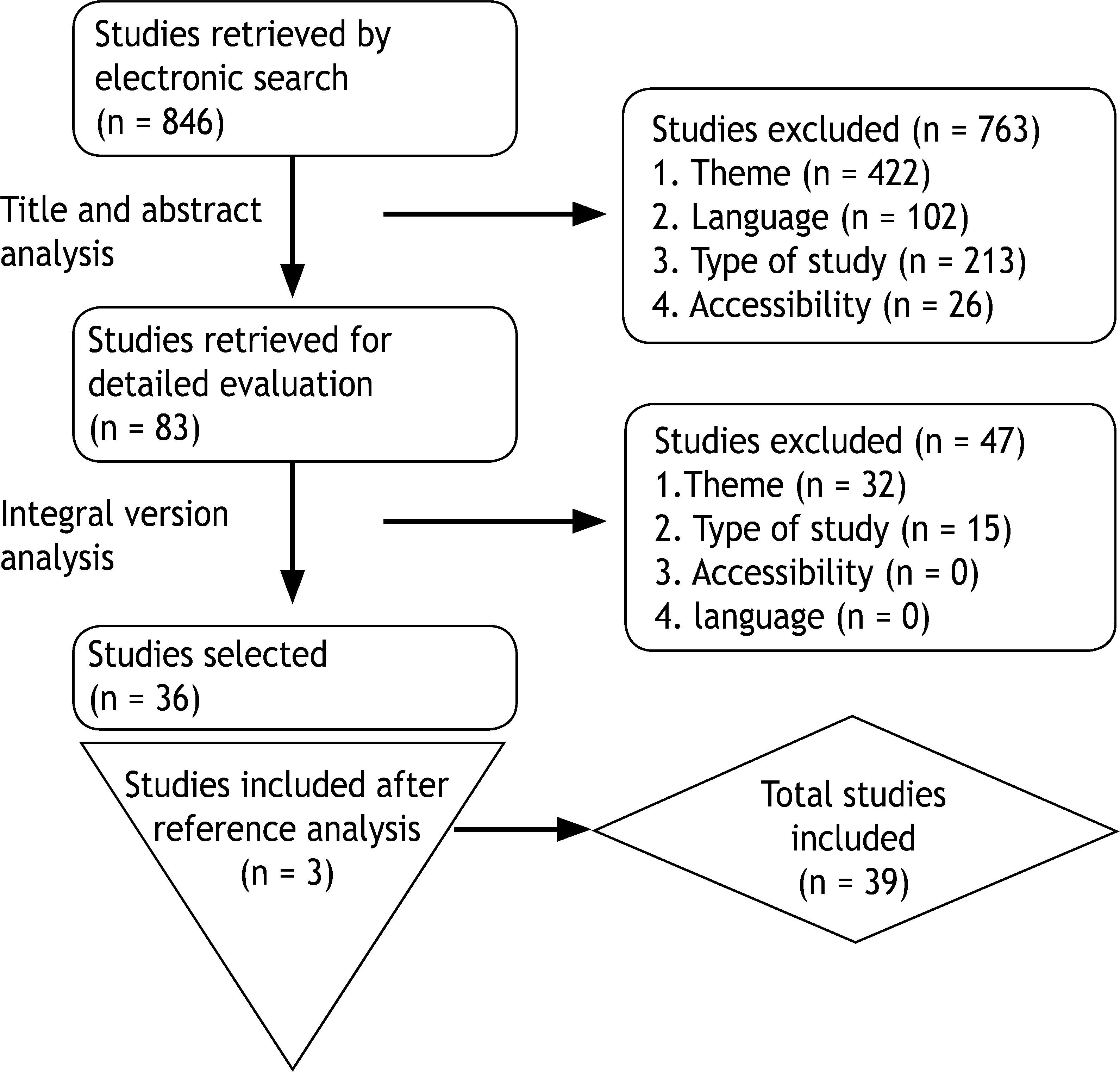
In the first stage, 846 studies were retrieved. After each article’s title and abstract were analyzed, 83 studies were selected. In the second phase, after the articles’ full analysis, 36 studies were selected. In each stage, the articles were assessed by two investigators and any divergence was solved by consensus. The two investigators worked independently and were blinded to each other’s assessments. Finally, after analyzing the reference list of all the selected studies, three new articles were included. A total of 39 studies were included in this systematic review and from each the following data was collected: title; author(s); publication date; journal; outcome definition and prevalence; study design; setting; sample size; association of risk factors with pharyngocutaneous fistula development; and time until outcome occurrence. The quality of the articles was assessed by the first author, following the Strengthening of the Reporting of Observational Studies in Epidemiology (STROBE) checklist for observational studies. This checklist has multiple components per item, resulting in a score of quality for observational studies.8 Two studies assessed risk classification systems.9,10 The study selection process is described in Fig. 1. The studies were first divided according to the intervention performed: total laryngectomy/primary pharyngolaryngectomy and “salvage surgery”. Then, they were ordered according to the design and quality assessment using the STROBE checklist (Table 1).
Flowchart of article selection.
The query used was: (postoperative complications[MeSH Terms]) OR postoperative complications[Title/Abstract]) OR postoperative complication[Title/ Abstract]) OR postoperative complication[MeSH Terms]) OR complications, postoperative[MeSH Terms]) OR complications, postoperative[Title/Abstract]) OR complication, postoperative[Title/Abstract]) OR complication, postoperative[MeSH Terms]) OR surgical wound dehiscence[MeSH Terms]) OR surgical wound dehiscence[Title/Abstract]) OR dehiscence surgical wound[Title/ Abstract]) OR dehiscence surgical wound[MeSH Terms]) OR anastomotic leaks[MeSH Terms]) OR anastomotic leaks[Title/Abstract]) OR anastomotic leakage[Title/Abstract]) OR anastomotic leakage[MeSH Terms]) OR anastomotic leakage[MeSH Terms]) OR anastomotic leakage[Title/Abstract]) OR healing wound[Title/Abstract]) OR healing wound[MeSH Terms]) OR fistula[MeSH Terms]) OR fistula[Title/Abstract]) OR pharyngocutaneous fistula[Title/Abstract]) OR pharyngocutaneous fistula[MeSH Terms]) OR cutaneous fistula[MeSH Terms]) OR cutaneous fistula[Title/Abstract]) OR skin fistula[Title/ Abstract]) OR skin fistula[MeSH Terms]) OR external fistula[MeSH Terms]) OR external fistula[Title/Abstract]) OR salivary gland fistula[Title/Abstract]) OR salivary gland fistula[MeSH Terms]) OR pharyngostoma[MeSH Terms]) OR pharyngostoma[Title/Abstract]) OR postla ryngectomy pharyngocutaneous fistula[Title/Abstract]) OR postlaryngectomy pharyngocutaneous fistula[MeSH Terms]) AND (factor risk[MeSH Terms]) OR factors risk[MeSH Terms]) OR risk factor[MeSH Terms]) OR risk factors[Title/Abstract]) OR causalities[Title/Abstract]) OR causalities[MeSH Terms]) OR multifactorial causality[MeSH Terms]) OR multifactorial causality[Title/Abstract]) OR predisposing factors[Title/ Abstract]) OR predisposing factors[MeSH Terms]) OR prognostic factors[MeSH Terms]) OR prognostic factors[Title/Abstract]) OR etiology[Title/Abstract]) OR etiology[MeSH Terms])) AND (laryngectomy[MeSH Terms]) OR laryngectomy[Title/ Abstract]) OR pharyngectomy[Title/Abstract]) OR pharyngectomy[MeSH Terms]) OR pharyngolaryngectomy[MeSH Terms]) OR pharyngolaryngectomy[Title/ Abstract]) OR laryngectomies[Title/Abstract]) OR laryngectomies[MeSH Terms]) OR neoplasms laryngeal[MeSH Terms]) OR cancer of larynx[MeSH Terms]) OR cancer of larynx[Title/Abstr act]) OR larynx cancer[Title/Abstract]) OR larynx cancer[MeSH Terms]) OR head neck cancer[MeSH Terms]) OR head neck cancer[Title/Abstract]) OR laryngeal cancer[Title/Abstract]) OR laryngeal cancer[MeSH Terms]) OR laryngopharyngectomy[MeSH Terms]) OR laryngopharyngectomy[Title/Abstract]).
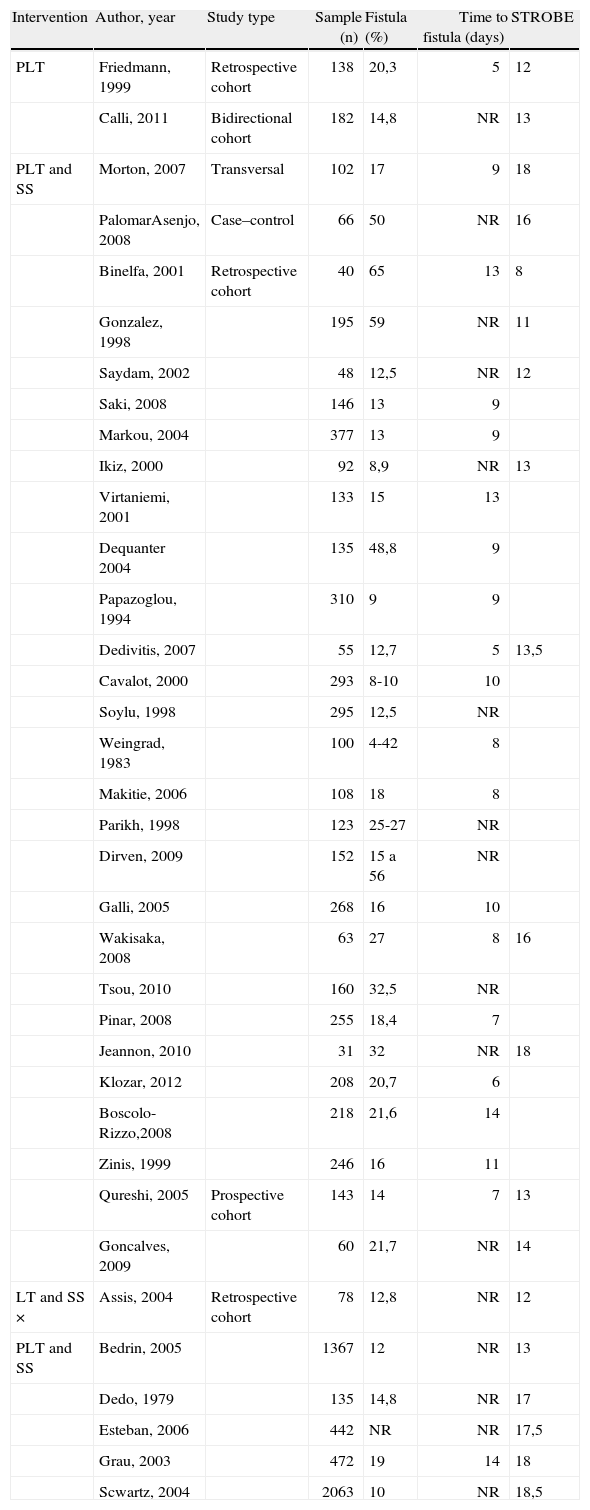
Characterization of the selected studies by type of intervention with STROBE classification and sample size.
| Intervention | Author, year | Study type | Sample (n) | Fistula (%) | Time to fistula (days) | STROBE |
| PLT | Friedmann, 1999 | Retrospective cohort | 138 | 20,3 | 5 | 12 |
| Calli, 2011 | Bidirectional cohort | 182 | 14,8 | NR | 13 | |
| PLT and SS | Morton, 2007 | Transversal | 102 | 17 | 9 | 18 |
| PalomarAsenjo, 2008 | Case–control | 66 | 50 | NR | 16 | |
| Binelfa, 2001 | Retrospective cohort | 40 | 65 | 13 | 8 | |
| Gonzalez, 1998 | 195 | 59 | NR | 11 | ||
| Saydam, 2002 | 48 | 12,5 | NR | 12 | ||
| Saki, 2008 | 146 | 13 | 9 | |||
| Markou, 2004 | 377 | 13 | 9 | |||
| Ikiz, 2000 | 92 | 8,9 | NR | 13 | ||
| Virtaniemi, 2001 | 133 | 15 | 13 | |||
| Dequanter 2004 | 135 | 48,8 | 9 | |||
| Papazoglou, 1994 | 310 | 9 | 9 | |||
| Dedivitis, 2007 | 55 | 12,7 | 5 | 13,5 | ||
| Cavalot, 2000 | 293 | 8-10 | 10 | |||
| Soylu, 1998 | 295 | 12,5 | NR | |||
| Weingrad, 1983 | 100 | 4-42 | 8 | |||
| Makitie, 2006 | 108 | 18 | 8 | |||
| Parikh, 1998 | 123 | 25-27 | NR | |||
| Dirven, 2009 | 152 | 15 a 56 | NR | |||
| Galli, 2005 | 268 | 16 | 10 | |||
| Wakisaka, 2008 | 63 | 27 | 8 | 16 | ||
| Tsou, 2010 | 160 | 32,5 | NR | |||
| Pinar, 2008 | 255 | 18,4 | 7 | |||
| Jeannon, 2010 | 31 | 32 | NR | 18 | ||
| Klozar, 2012 | 208 | 20,7 | 6 | |||
| Boscolo-Rizzo,2008 | 218 | 21,6 | 14 | |||
| Zinis, 1999 | 246 | 16 | 11 | |||
| Qureshi, 2005 | Prospective cohort | 143 | 14 | 7 | 13 | |
| Goncalves, 2009 | 60 | 21,7 | NR | 14 | ||
| LT and SS × | Assis, 2004 | Retrospective cohort | 78 | 12,8 | NR | 12 |
| PLT and SS | Bedrin, 2005 | 1367 | 12 | NR | 13 | |
| Dedo, 1979 | 135 | 14,8 | NR | 17 | ||
| Esteban, 2006 | 442 | NR | NR | 17,5 | ||
| Grau, 2003 | 472 | 19 | 14 | 18 | ||
| Scwartz, 2004 | 2063 | 10 | NR | 18,5 |
PLT, pharyngolaryngectomy; LT, total laryngectomy; SS, “salvage surgery”; NR, not related.
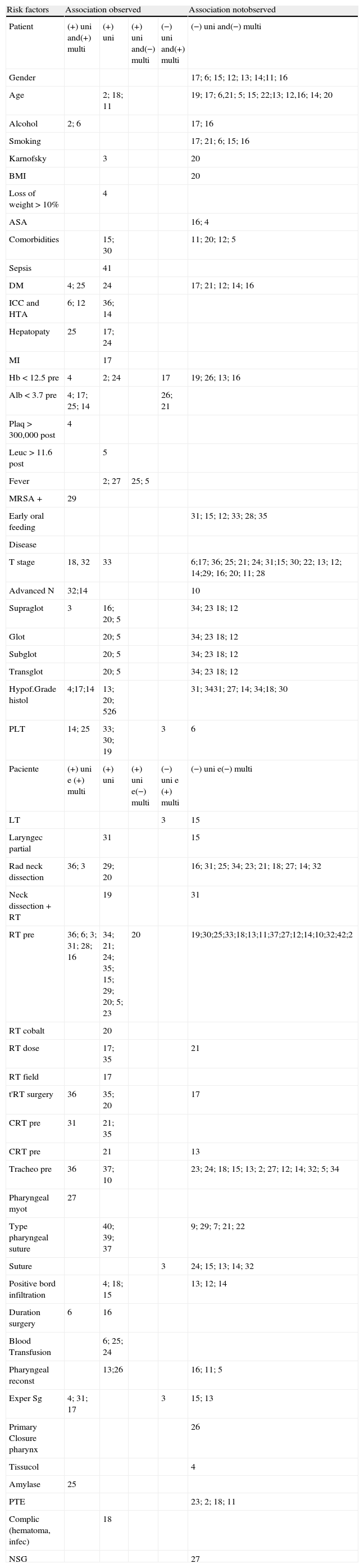
Thereafter, all risk factors evaluated were extracted and listed individually by the type of statistical analysis/ methodology performed in each study, and also in relation to positive or negative association for the development of the pharyngocutaneous fistula (Table 2). The final analysis and synthesis of risk factors for development of pharyngo- cutaneous fistula is shown in Table 3.
Observed association between the presence of risk factors and evelopment of pharyngocutaneous fistula with the univariate and multivariate analysis: (+) uni, observed association with univariate analysis and the multivariate analysis was not performed; (+) uni and (+) multi, an association was observed between the resence of risk factors and development of the pharyngocutaneous fistula in the univariate and multivariate analyzes; (+) uni and (−) multi, observed association in univariate analysis and it was not observed in multivariate analysis; (−) uni and (+) multi, an association was observed in the multivariate analysis and was not observed in the univariate analysis; (−) uni and (−) multi, no association was observed in the univariate and multivariate analysis.
| Risk factors | Association observed | Association notobserved | |||
| Patient | (+) uni and(+) multi | (+) uni | (+) uni and(−) multi | (−) uni and(+) multi | (−) uni and(−) multi |
| Gender | 17; 6; 15; 12; 13; 14;11; 16 | ||||
| Age | 2; 18; 11 | 19; 17; 6,21; 5; 15; 22;13; 12,16; 14; 20 | |||
| Alcohol | 2; 6 | 17; 16 | |||
| Smoking | 17; 21; 6; 15; 16 | ||||
| Karnofsky | 3 | 20 | |||
| BMI | 20 | ||||
| Loss of weight >10% | 4 | ||||
| ASA | 16; 4 | ||||
| Comorbidities | 15; 30 | 11; 20; 12; 5 | |||
| Sepsis | 41 | ||||
| DM | 4; 25 | 24 | 17; 21; 12; 14; 16 | ||
| ICC and HTA | 6; 12 | 36; 14 | |||
| Hepatopaty | 25 | 17; 24 | |||
| MI | 17 | ||||
| Hb<12.5 pre | 4 | 2; 24 | 17 | 19; 26; 13; 16 | |
| Alb<3.7 pre | 4; 17; 25; 14 | 26; 21 | |||
| Plaq>300,000 post | 4 | ||||
| Leuc>11.6 post | 5 | ||||
| Fever | 2; 27 | 25; 5 | |||
| MRSA + | 29 | ||||
| Early oral feeding | 31; 15; 12; 33; 28; 35 | ||||
| Disease | |||||
| T stage | 18, 32 | 33 | 6;17; 36; 25; 21; 24; 31;15; 30; 22; 13; 12; 14;29; 16; 20; 11; 28 | ||
| Advanced N | 32;14 | 10 | |||
| Supraglot | 3 | 16; 20; 5 | 34; 23 18; 12 | ||
| Glot | 20; 5 | 34; 23 18; 12 | |||
| Subglot | 20; 5 | 34; 23 18; 12 | |||
| Transglot | 20; 5 | 34; 23 18; 12 | |||
| Hypof.Grade histol | 4;17;14 | 13; 20; 526 | 31; 3431; 27; 14; 34;18; 30 | ||
| PLT | 14; 25 | 33; 30; 19 | 3 | 6 | |
| Paciente | (+) uni e (+) multi | (+) uni | (+) uni e(−) multi | (−) uni e (+) multi | (−) uni e(−) multi |
| LT | 3 | 15 | |||
| Laryngec partial | 31 | 15 | |||
| Rad neck dissection | 36; 3 | 29; 20 | 16; 31; 25; 34; 23; 21; 18; 27; 14; 32 | ||
| Neck dissection+RT | 19 | 31 | |||
| RT pre | 36; 6; 3; 31; 28; 16 | 34; 21; 24; 35; 15; 29; 20; 5; 23 | 20 | 19;30;25;33;18;13;11;37;27;12;14;10;32;42;2 | |
| RT cobalt | 20 | ||||
| RT dose | 17; 35 | 21 | |||
| RT field | 17 | ||||
| t'RT surgery | 36 | 35; 20 | 17 | ||
| CRT pre | 31 | 21; 35 | |||
| CRT pre | 21 | 13 | |||
| Tracheo pre | 36 | 37; 10 | 23; 24; 18; 15; 13; 2; 27; 12; 14; 32; 5; 34 | ||
| Pharyngeal myot | 27 | ||||
| Type pharyngeal suture | 40; 39; 37 | 9; 29; 7; 21; 22 | |||
| Suture | 3 | 24; 15; 13; 14; 32 | |||
| Positive bord infiltration | 4; 18; 15 | 13; 12; 14 | |||
| Duration surgery | 6 | 16 | |||
| Blood Transfusion | 6; 25; 24 | ||||
| Pharyngeal reconst | 13;26 | 16; 11; 5 | |||
| Exper Sg | 4; 31; 17 | 3 | 15; 13 | ||
| Primary Closure pharynx | 26 | ||||
| Tissucol | 4 | ||||
| Amylase | 25 | ||||
| PTE | 23; 2; 18; 11 | ||||
| Complic (hematoma, infec) | 18 | ||||
| NSG | 27 | ||||
BMI, body mass index; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; ICC, congestive cardiac disease; HTA, arterial hypertension; MI, myocardial infarction; Hb, hemoglobin; Alb, albumin; Plaq: platelets; Leuc, leucocits; MRSA, methicillinresistant Staphylococcus aureus; Supraglot, supraglottic; Glot, glottic; subglot, subglottic; Transglot, transglottic; Hipof, hypopharynx; histol, histological; PLT, pharyngolaryngectomy; LT, total laryngectomy; Laryngec, laryngectomy; Rad, radical; RT, radiotherapy; t´RT, interval time betwEen RT and surgery; CRT, chemoradiotherapy; CT, chemotherapy; traqueo, tracheotomy; myot, myotomy; reconst, reconstruction; Exper. Sg, surgeon experience; PTE, tracheoesophagic puncture; Complic, complications; infec, infection; NSG, nasogastric tube; 1 to 43, references number
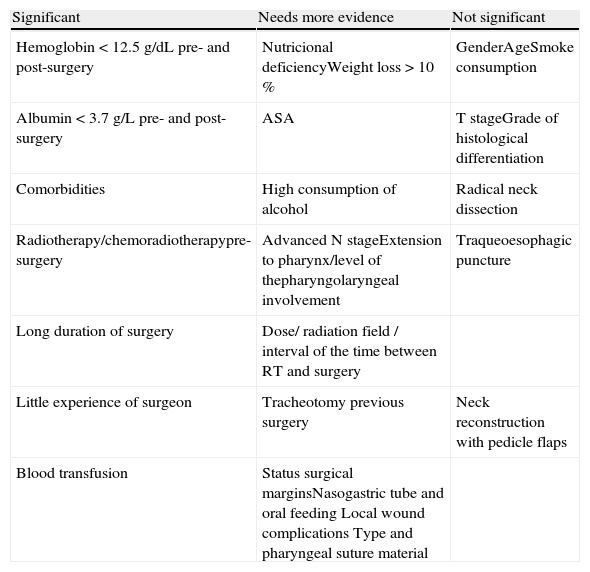
Summary of studied risk factors for pharyngocutaneous fistula development.
| Significant | Needs more evidence | Not significant |
| Hemoglobin<12.5g/dL pre- and post-surgery | Nutricional deficiencyWeight loss>10 % | GenderAgeSmoke consumption |
| Albumin<3.7g/L pre- and post-surgery | ASA | T stageGrade of histological differentiation |
| Comorbidities | High consumption of alcohol | Radical neck dissection |
| Radiotherapy/chemoradiotherapypre-surgery | Advanced N stageExtension to pharynx/level of thepharyngolaryngeal involvement | Traqueoesophagic puncture |
| Long duration of surgery | Dose/ radiation field / interval of the time between RT and surgery | |
| Little experience of surgeon | Tracheotomy previous surgery | Neck reconstruction with pedicle flaps |
| Blood transfusion | Status surgical marginsNasogastric tube and oral feeding Local wound complications Type and pharyngeal suture material |
In two studies the intervention was pharyngolaryngectomy; in 29 was pharyngolaryngectomy was compared with salvage surgery; and in six, total laryngectomy and salvage surgery was compared with pharyngolaryngectomy salvage surgery. Two studies described and evaluated pharyngocutaneous fistula development risk classification systems. Regarding the study design, one was a case–control, one was a bidirectional cohort, two were prospective cohorts, one was a transversal cohort and 32 were retrospective cohorts. Not many prospective studies were retrieved in this review. In the STROBE checklist, values ranged from 8 to 18.5, with a mean score of 14.5. Sample size varied between 31 to 2,063 subjects, and 89% of studies were single-center. The fistula incidence varied from 4% to 65%.
Analysis of risk factorsRelated to the patientCharacterization of patientsAmong the inherent characteristics of the patients, gender was not associated with pharyngocutaneous fistula formation in any of the studies retrieved,6,11–17 and the male gender was always more frequent in the studies’ samples. Age was associated with fistula in some studies,2,11,18 although not in all.5,6,11–17,19–22 Esteban et al.2 and Galli et al.6 confirmed the association between high alcohol consumption and fistulization. However, in other studies,16,17 this finding did not achieve statistical significance. Smoking habits were not considered to be a significant risk factor for the development of pharyngocutaneous fistula in most studies.6,15–17,21 Nutritional deficiency and weight loss greater than 10% in the six months prior to surgery still need to be further researched, since only two studies considered these factors as significant for fistula;3,4 one study did not observe a significant association.20
Regarding the American Society of Anesthesiologists Risk Classification System(ASA scale), some authors4,16,23 have evaluated its capacity for predicting the development of pharyngocutaneous fistula and found no significant association in their sample of 246 and 2,063 patients, respectively.
Analytical parametersHemoglobin and serum albumin level, platelet count and the number of leukocytes are much discussed in the literature as possible risk factors for the development of pharyngocutaneous fistula. Postoperative leukocytosis (>11.6) (×109/L) and thrombocytosis(>300)(×109/L) were considered by Makitie et al.4 and by Schwartz et al.5 as significant factors for fistulization in a sample of 2,171 patients. None of the other studies retrieved considered these analytical parameters. Low hemoglobin (<12.5g/dL) and hypoalbuminemia (<3.7g/L), both preoperative and postoperative, have been mentioned by some authors as major predictive factors for fistula formation and development: Boscolo-Rizzo et al. Cavalot et al., Esteban et al., Pinar et al., Schwartz et al., and Tsou et al.,2,4,14,17,24,25 with a total sample of 3,420 patients and mean STROBE of 16. Other authors13,16,19,21,26 did not identify such association when studying a total sample of 620 patients with a mean STROBE score of 16.
ComorbiditiesMany comorbidities presented by patients have been reported as possible risk factors for formation of pharyngocutaneous fistula. Acute myocardial infarction (AMI) was mentioned by Tsou et al.17 as a significant factor in their univariate analysis for fistula development. Liver diseases, such as hepatic cirrhosis, were observed to be associated with fistula in a total sample of 671 patients.17,24,25 Hypertension was associated with pharyngocutaneous fistula development in some studies6,14,16 with a total sample of 769 patients. Chronic obstructive pulmonary disease (COPD) was considered by Boscolo-Rizzo et al.25 as a significant factor for fistulization, in an analysis of 218 patients; however, Pinar et al. and Redaelli Zinis et al.14,16 did not observe such association in their analysis including 501 patients. Diabetes mellitus (DM) was significantly associated with fistula development,4,24,25 with a total sample of 2,500 patients and a mean score of STROBE of 16.8. Other studies have not demonstrated a significant relation between fistula formation and comorbidities,12,14,16,7,21 in a total sample of 845 patients with mean STROBE score of 16.
FeverThe presence of postoperative fever was considered to be an early sign for pharyngocutaneous fistula formation by some authors,5,25,27 but it should be interpreted with caution after excluding other possible fever causes in the immediate postoperative period.
Related to diseaseTMN stage and grade of tumor differentiationFocusing on this subject, 17 authors did not consider late stage of tumors as a significant factor for the formation of pharyngocutaneous fistula. The total sample was 3,036 patients with a mean STROBE score of 15.6,11–17,22,20,21,25,24,28–31 Three studies (Grau et al., Klozar et al., and Soylu et al.) 18,32,33 observed that advanced T stage was associated with fistula development in a sample of 975 patients and a mean STROBE score of 13.
Advanced N stage was considered to be a significant risk factor in two studies,14,32 with a total sample of 463 patients and STROBE score of 17. Only the study by Parikh et al.12 considered it to not be associated with fistula.
In the study by Friedman et al.27 the degree of histological differentiation of the tumors was associated with outcome occurrence in a sample of 138 patients with a STROBE score of 12. Other authors16,28,31,32 observed no causality between the factor analyzed and fistula development.
Treatment-relatedExtended surgery to the pharynxAmong all the studies reviewed, five authors reported a causal relationship for pharyngocutaneous fistula development in univariate and multivariate analysis in their samples regarding the surgical extension to the pharynx.16,19,26,32,34 These studies showed a sample of 560 patients and a mean STROBE score of 15.8. The study by Galli et al.6 with 268 patients observed a significant relationship in the multivariate analysis between the surgical option and the development of the pharyngocutaneous fistula. However, Schwartz et al.,4 in a multicenter study of 2,063 patients with STROBE score of 18.5, did not find a significant relationship between the formation of the pharyngocutaneous fistula and the extent of surgery to the pharynx. Regarding tumor location and the level of laryngopharyngeal commitment, eight studies evaluated as a positive factor the location and extent of the tumor (glottis, subglottis, supraglottis, hypopharynx) for the postoperative development of fistula, with a sample of 1,760 patients and mean STROBE score of 15.2,3,6,13,16–18,20 However, other studies (total sample of 1,800 patients and mean STROBE score of 14.5) did not observe a significant relationship between these variables and fistulization.5,11,14,15,22,21,25,30,32
Radical neck dissectionRadical neck dissection was considered to be significant for the development of the pharyngocutaneous fistula in four studies,6,20,30,35 with a total sample of 840 patients and mean STROBE score of 14.5. Conversely, ten studies with a total sample of 2,200 patients and mean STROBE score of 16 observed no significant relationship between radical neck dissection and the occurrence of fistula. 16,17,21,22,25,26,28,32,33,36
Preoperative radiotherapy (RT) or/chemoradiothe-rapy (CRT)Preoperative RT or CRT are controversial issues among authors. 54% (20) of the analyzed studies observed that preoperative RT had a strong relationship with the development of pharyngocutaneous fistulas (16% of the studies performed a univariate and a multivariate analysis, and in 37.8%, the result was obtained by univariate analysis of the sample). Regarding sample size, studies with positive association between radiotherapy and fistula formation included 31 to 2,063 patients, and the STROBE score ranged from 8 to 18, with a mean score of 17.8. The combined regimen of CRT performed prior to surgery presented a significant relationship with the development of fistula in three studies.21,32,37
Regarding the studies with a negative association between RT and the development of fistula,the sample size varied from 48 to 377 patients, and STROBE score ranged from 11 to 18, with mean score of 14.3. Studies with larger samples and those with higher STROBE score showed a significant relationship between RT and CRT for the development of the pharyngocutaneous fistula. While most studies have evaluated the dose/radiation field, only two studies18,37 observed a significant relationship between radiotherapy doses and radiation fields for postoperative fistula development, with samples of 472 and 152 patients and STROBE scores of 18 and 15, respectively. Both studies performed only univariate analysis. Only one study including 63 patients21 observed a non-significant relationship between radiation dose and the development of the pharyngocutaneous fistula.
The time interval between RT and surgery was found to be significant for the early development of fistula (less than three months) in three studies20,37,35 with a total sample of 420 patients and a STROBE score ranging from 13 to 17; only one study performed a multivariate analysis with this factor.35 Grau et al.,18 in a multicenter study with univariate and multivariate analysis, were the only authors to observe a non significant relationship between the time interval from RT to surgery and fistula formation in a sample of 472 patients with a STROBE score of 18.
Previous tracheotomyMost studies3,5,14,13,15,16,22,24,25,28,33 did not observe a relationship between emergency tracheotomy and development of the pharyngocutaneous fistula. Three studies (Dedivitis et al.,11 Gonzalez Aguilar et al.,38 and Horgan & Dedo et al.35) with a total sample of 384 patients and mean STROBE score of 14 observed an association between the variable and the outcome.
Type of suture materialMost studies did not consider the type of suture material used for the pharynx closure as a significant factor to the formation of the pharyngocutaneous fistula.11,13,15,16,24,33,39–41 Other authors38,40,41 pointed to the superiority of mechanical suture over the manual suture for closure of the pharynx, with a lower incidence of fistula formation in their total sample of 437 patients. Primary closure of the pharynx and the use of Tissucol as adjuvant have been cited by some authors (Esteban et al., Friedman et al.,),2,27 but no significant association with pharyngocutaneous fistula development was observed in a sample of 580 patients with mean STROBE score of 14. The need for pharyngeal reconstruction significantly increased the likelihood of fistulization in the studies by Friedman et al. and for Qureshi et al.,13,27 namely using flaps, such as the pectoralis major myocutaneous flap, in a total sample of 280 patients with a mean STROBE score of 12.5; unlike other authors,3,12,17 who observed non-significant results in a sample of 420 patients with mean STROBE score of 14.
Status of surgical marginsMicroscopic histological infiltration by the tumor in the surgical margins has been described by some authors2,15,22 as statistically significant for the development of pharyngocu- taneous fistula in a total sample of 965 patients with a mean STROBE score of 14. However, other authors did not find a significant relationship in a analysis of a sample of 645 patients with mean STROBE score of 15.13,14,16
Surgical procedureRegarding surgical treatment, the long duration of the surgery and the need for blood transfusion during surgery were considered as risk factors for fistula formation by some authors4,17,24,26 in a total sample of 2,618 patients with mean STROBE score of 16.7. The implementation of tracheoesophageal puncture for insertion of voice prosthesis in the ini - tial procedure was not a significant factor in the development of the fistula in other studies.5,12,22,25
Less surgical experience was reported in some studies as a significant factor in the formation of the pharyngocu- taneous fistula.2,6,18,32 These studies presented a sample of 1,400 patients with a mean STROBE score of 17.
Local wound complicationsIn three studies,22,26,29 local complications of the wound, such as methicillin-resistent Staphylococcus aureus (MRSA) infection, wound hematoma, and the presence of amylase in the cervical drainage were significant factors for postoperative fistulization. Jeannon et al.29 presented a sample of 31 patients. Markou et al.,22 had a sample of 377 patients with STROBE score of 12. Morton et al.26 were the only authors to demonstrate that the presence of amylase in the drains was a predictive factor for the development of pharyngocutaneous fistula in a sample of 102 patients with a mean STROBE score of 18.
Nasogastric tube (NGT) and oral feedingMany studies4,12,15,28,31,33,35 have observed that the absence of the nasogastric tube or its removal without replacement with premature early oral feeding (less than 14 days after operation) does not cause an increase in the rate of formation of the fistula. However, more evidence is needed.
Scales of risk assessmentIn this review, two further studies were included proposing two classifications concerning predictive analysis of risk factors for morbidity and perioperative complications: Lancaster et al., using the Physiological and Operative Severity Score for the Enumeration of Mortality and Morbidity (POSSUM) scale, and Farwell et al., using the ASA classification.9,10
The POSSUM scale has many limitations regarding its applicability in head and neck oncologic surgery. Peritoneal contamination is not relevant in this context, and the different surgical specialties with their different magnitudes are not well defined as the score awarded. Risk factors related to local surgical complications have been widely discussed in the literature and are not among the variables, for example, RT and CRT prior to surgery, T stage, neck dissection, tracheotomy prior surgery, nutritional status, and co-existence of systemic disease. There is no assessment of patient nutritional status and analytical level of albumin. The scale does not define values for the complications presented, was not primarily designed as a predictor of surgical complications (overall morbidity) for head and neck, and it is not specific for pharyngocutaneous fistula. The authors concluded that the POSSUM scale has several limitations for application in laryngectomized patients due to the omission of important factors related to the outcomes evaluated. They9 concluded that the POSSUM scale cannot correctly identify the group of patients that could develop pharyngocutaneous fistula, and emphasized the need to create a scale specific to head and neck cancer surgery.
According to Farwell et al. 2002, the ASA classification is used to evaluate co-morbidity, since it describes the current physical state of patients prior to surgery. It is currently the standard scale to measure preoperative risk by anesthesiologists. Its limitation lies in the fact that it addresses the health status of the patient on the day of the surgery, without predictive power for the complications associated with the surgery performed. It is also limited by the fact that the majority of the patients with head and neck cancer present with multiple morbidities and generally have ASA class III to V.10Although the selected study10 was not specific for patients submitted to laryngectomy and the development of pharyngocutaneous fistula, it included because it had appropriate methodology and discriminated the results of medical and surgical complications. The authors concluded that the ASA classification was not significant for the prediction of complications in their series of patients, since most of them showed multiple medical morbidities and were classified as class II or III. The study did not include diagnostic measures.
DiscussionAnalyzing the systematic review, it is clear that there is still no consensus among studies on the role and relevance of each risk factor for the development of pharyngocutaneous fistulas. It is also clear that, depending on the population studied, some factors are more significant and consistent than others.
A single meta-analysis developed in the literature for pharyngocutaneous fistula after laryngectomy was performed by Paydarfar et al.42 and observed that postoperative hemoglobin less than 12.5g/dL, tracheotomy performance prior to surgery and radiotherapy previous to surgery with or without neck dissection were the most significant risk factors for the development of pharyngocutaneous fistula after total laryngectomy. However, the authors were limited to primary cancer of the larynx and did not include any study that addressed the treatment of primary neoplasm of the hypopharynx. While it may represent a selection bias, it is believed that the inclusion of studies whose intervention goes beyond the standard laryngectomy, is including partial laryngectomy with pharyngectomy is relevant, since the results of these studies should also be evaluated and reported with regard to their weight and their importance to the development of the pharyngocutaneous fistula.
In the present review, Esteban et al.2 developed a model using logistic analysis to assess risk factors for the development of pharyngocutaneous fistula in 442 patients who underwent total laryngectomy. The authors included variables before, during, and after surgery, such as the amount of alcohol consumption, lack of involvement of the pyriform sinus and base of the tongue, surgeon’s experience, the use of biological glue during surgery and the presence or absence of fever in the postoperative period. However, the model proposed did not provide diagnostic measures, such as calculations for sensitivity, specificity, positive and negative predictive values and area under the receiver operating characteristic (ROC) curve. Furthermore, the model cannot be applied to the present patients, since it involved a specific surgeon working at the university hospital of the study population and the use or nonuse of Tissucol for calculating the risk of fistula development, factors inherent in this very specific study.
The present systematic review also had limitations: the exclusion of studies whose translation was not possible; the assessment of trial quality and the fact that data extraction was performed only by the main author. The absence of prospective studies in the literature is noteworthy.
In summary, despite the difficulty in reaching a consensus regarding the most important risk factors for the development of the pharyngocutaneous fistula, this review considered studies with larger samples and with better quality in the overall assessment by the STROBE scale, as they are able to show greater evidence and the importance of some risk factors. Likewise, some factors were considered to be non-significant in the development of fistula and other factors need further analysis to demonstrate evidence and their role in this aspect (Table 3).
It can be concluded that there is still no appropriate classification for stratifying the risk of appearance of pharyngo- cutaneous fistulas. Therefore, the selection and synthesis of the most significant risk factors for fistula development were: hemoglobin pre- and post-surgery<12.5g/dL; pre- and postsurgery albumin<3.7g/L; presence of comorbidities; performance of previous RT or CRT; long duration of surgery; blood transfusion during operation and little experience of the main surgeon. Finally, the need for further studies with larger sample size, multicenter design and adequate methodology is emphasized, in order to obtain scientific evidence and consolidate the knowledge on the subject.
It is also necessary to create new levels of risk stratification that are appropriate to the different realities and greater efforts by experts in the evaluation and study of all possible variables related to fistulization. Only in this way can the occurrence of postoperative complications, which are so painful for the patient and the surgeon, be successfully avoided.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Cecatto SB, Soares MM, Henriques T, Monteiro E, Moura CIFP. Predictive factors for the postlaryngectomy pharyngocutaneous fistula development: systematic review. Braz J Otorhinolaryngol. 2014;80:167–77.
Study conducted as part of a MSc research, and it was presented by the main author as a MSc dissertation in Evidence-based Medicine,at Faculdade de Medicina, Universidade do Porto, Portugal, in November 2012



![Flowchart of article selection. The query used was: (postoperative complications[MeSH Terms]) OR postoperative complications[Title/Abstract]) OR postoperative complication[Title/ Abstract]) OR postoperative complication[MeSH Terms]) OR complications, postoperative[MeSH Terms]) OR complications, postoperative[Title/Abstract]) OR complication, postoperative[Title/Abstract]) OR complication, postoperative[MeSH Terms]) OR surgical wound dehiscence[MeSH Terms]) OR surgical wound dehiscence[Title/Abstract]) OR dehiscence surgical wound[Title/ Abstract]) OR dehiscence surgical wound[MeSH Terms]) OR anastomotic leaks[MeSH Terms]) OR anastomotic leaks[Title/Abstract]) OR anastomotic leakage[Title/Abstract]) OR anastomotic leakage[MeSH Terms]) OR anastomotic leakage[MeSH Terms]) OR anastomotic leakage[Title/Abstract]) OR healing wound[Title/Abstract]) OR healing wound[MeSH Terms]) OR fistula[MeSH Terms]) OR fistula[Title/Abstract]) OR pharyngocutaneous fistula[Title/Abstract]) OR pharyngocutaneous fistula[MeSH Terms]) OR cutaneous fistula[MeSH Terms]) OR cutaneous fistula[Title/Abstract]) OR skin fistula[Title/ Abstract]) OR skin fistula[MeSH Terms]) OR external fistula[MeSH Terms]) OR external fistula[Title/Abstract]) OR salivary gland fistula[Title/Abstract]) OR salivary gland fistula[MeSH Terms]) OR pharyngostoma[MeSH Terms]) OR pharyngostoma[Title/Abstract]) OR postla ryngectomy pharyngocutaneous fistula[Title/Abstract]) OR postlaryngectomy pharyngocutaneous fistula[MeSH Terms]) AND (factor risk[MeSH Terms]) OR factors risk[MeSH Terms]) OR risk factor[MeSH Terms]) OR risk factors[Title/Abstract]) OR causalities[Title/Abstract]) OR causalities[MeSH Terms]) OR multifactorial causality[MeSH Terms]) OR multifactorial causality[Title/Abstract]) OR predisposing factors[Title/ Abstract]) OR predisposing factors[MeSH Terms]) OR prognostic factors[MeSH Terms]) OR prognostic factors[Title/Abstract]) OR etiology[Title/Abstract]) OR etiology[MeSH Terms])) AND (laryngectomy[MeSH Terms]) OR laryngectomy[Title/ Abstract]) OR pharyngectomy[Title/Abstract]) OR pharyngectomy[MeSH Terms]) OR pharyngolaryngectomy[MeSH Terms]) OR pharyngolaryngectomy[Title/ Abstract]) OR laryngectomies[Title/Abstract]) OR laryngectomies[MeSH Terms]) OR neoplasms laryngeal[MeSH Terms]) OR cancer of larynx[MeSH Terms]) OR cancer of larynx[Title/Abstr act]) OR larynx cancer[Title/Abstract]) OR larynx cancer[MeSH Terms]) OR head neck cancer[MeSH Terms]) OR head neck cancer[Title/Abstract]) OR laryngeal cancer[Title/Abstract]) OR laryngeal cancer[MeSH Terms]) OR laryngopharyngectomy[MeSH Terms]) OR laryngopharyngectomy[Title/Abstract]). Flowchart of article selection. The query used was: (postoperative complications[MeSH Terms]) OR postoperative complications[Title/Abstract]) OR postoperative complication[Title/ Abstract]) OR postoperative complication[MeSH Terms]) OR complications, postoperative[MeSH Terms]) OR complications, postoperative[Title/Abstract]) OR complication, postoperative[Title/Abstract]) OR complication, postoperative[MeSH Terms]) OR surgical wound dehiscence[MeSH Terms]) OR surgical wound dehiscence[Title/Abstract]) OR dehiscence surgical wound[Title/ Abstract]) OR dehiscence surgical wound[MeSH Terms]) OR anastomotic leaks[MeSH Terms]) OR anastomotic leaks[Title/Abstract]) OR anastomotic leakage[Title/Abstract]) OR anastomotic leakage[MeSH Terms]) OR anastomotic leakage[MeSH Terms]) OR anastomotic leakage[Title/Abstract]) OR healing wound[Title/Abstract]) OR healing wound[MeSH Terms]) OR fistula[MeSH Terms]) OR fistula[Title/Abstract]) OR pharyngocutaneous fistula[Title/Abstract]) OR pharyngocutaneous fistula[MeSH Terms]) OR cutaneous fistula[MeSH Terms]) OR cutaneous fistula[Title/Abstract]) OR skin fistula[Title/ Abstract]) OR skin fistula[MeSH Terms]) OR external fistula[MeSH Terms]) OR external fistula[Title/Abstract]) OR salivary gland fistula[Title/Abstract]) OR salivary gland fistula[MeSH Terms]) OR pharyngostoma[MeSH Terms]) OR pharyngostoma[Title/Abstract]) OR postla ryngectomy pharyngocutaneous fistula[Title/Abstract]) OR postlaryngectomy pharyngocutaneous fistula[MeSH Terms]) AND (factor risk[MeSH Terms]) OR factors risk[MeSH Terms]) OR risk factor[MeSH Terms]) OR risk factors[Title/Abstract]) OR causalities[Title/Abstract]) OR causalities[MeSH Terms]) OR multifactorial causality[MeSH Terms]) OR multifactorial causality[Title/Abstract]) OR predisposing factors[Title/ Abstract]) OR predisposing factors[MeSH Terms]) OR prognostic factors[MeSH Terms]) OR prognostic factors[Title/Abstract]) OR etiology[Title/Abstract]) OR etiology[MeSH Terms])) AND (laryngectomy[MeSH Terms]) OR laryngectomy[Title/ Abstract]) OR pharyngectomy[Title/Abstract]) OR pharyngectomy[MeSH Terms]) OR pharyngolaryngectomy[MeSH Terms]) OR pharyngolaryngectomy[Title/ Abstract]) OR laryngectomies[Title/Abstract]) OR laryngectomies[MeSH Terms]) OR neoplasms laryngeal[MeSH Terms]) OR cancer of larynx[MeSH Terms]) OR cancer of larynx[Title/Abstr act]) OR larynx cancer[Title/Abstract]) OR larynx cancer[MeSH Terms]) OR head neck cancer[MeSH Terms]) OR head neck cancer[Title/Abstract]) OR laryngeal cancer[Title/Abstract]) OR laryngeal cancer[MeSH Terms]) OR laryngopharyngectomy[MeSH Terms]) OR laryngopharyngectomy[Title/Abstract]).](https://static.elsevier.es/multimedia/18088694/0000008000000002/v2_201407291119/S1808869414500444/v2_201407291119/pt/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/7hLX2FbBoxC1192i158SI=)


