Medication-related osteonecrosis of the jaws is a severe complication of the use of antiresorptive and antiangiogenic therapy, with limited treatment options and great impact on patient’s quality pf life.
ObjectiveThe aim of this study was to assess the risk factors associated with medication-related osteonecrosis of the jaws in oncologic patients undergoing bisphosphonate treatment. In addition, salivary levels of interleukin-6, IL-6, were measured to investigate their association with severity and risk of medication-related osteonecrosis of the jaws.
MethodsCase-control study with 74 patients with bone metastases from solid tumors and multiple myeloma was included. Patients were divided into three groups: 1) those undergoing bisphosphonate treatment with medication-related osteonecrosis of the jaws; 2) those undergoing bisphosphonate without medication-related osteonecrosis of the jaws; and 3) those with bisphosphonate pretreatment. The demographic and medical data of the patients were collected to assess risk. The clinical evaluation was performed to diagnose medication-related osteonecrosis of the jaws and unstimulated saliva was collected for quantification of IL-6.
ResultsAs result, it was observed that patients diagnosed with medication-related osteonecrosis of the jaws were submitted to higher number of bisphosphonate doses (p = 0.001) and monthly infusion protocol (p = 0.044; OR = 7.75). Patients who did not have routine followup with specialized dentists during therapy with bisphosphonate and smoking were associated with medication-related osteonecrosis of the jaws (p = 0.019; OR = 8.25 and p = 0.031; OR = 9.37 respectively). Group 1 had a higher frequency of treatment with chemotherapy and corticosteroids concomitant with bisphosphonate, and surgical dental procedures (p = 0.129). Salivary IL-6 levels showed no statistically significant difference between the groups (p = 0.571) or association with medication-related osteonecrosis of the jaws severity (p = 0.923).
ConclusionA higher number of bisphosphonate cycles, monthly infusion protocol, no dental follow-up for oral health maintenance and smoking were associated with medication-related osteonecrosis of the jaws. Specialized dental follow up during bisphosphonate treatment has been shown to be an important factor in preventing this complication.
Bisphosphonates (BP) are antiresorptive agents indicated for the treatment of skeletal complications associated with multiple myeloma and bone metastases from solid tumors, in addition to the treatment of osteoporosis and osteopenia.1,2 Despite its clinical benefit, one of its side effects is medication- related osteonecrosis of the jaw (MRONJ), which is a serious complication, with a direct impact on patients' quality of life and oncologic treatment.2,3
Medication- related osteonecrosis of the jaw is defined as the presence of exposed bone or bone that can be probed through an intra- or extra-oral fistula in the maxillofacial region with persistence of more than 8 weeks, absence of radiotherapy or metastatic disease in the jaws of patients in current or previous treatment with antiresorptive or antiangiogenics.4 Symptoms may include pain, swelling, erythema and tooth loss associated with infections.5
The incidence of intravenous BP in cancer patients ranges from 1.2% to 9.9%. The highest frequency is associated with multiple myeloma and lowest in breast cancer patients.2
The pathophysiology of MRONJ has not been fully elucidated.4,6 It is believed that its occurrence caused by BP begin with pH reduction after oral infection or dental surgery that cause release and cause activation of toxic BP levels.2 BPs have anti-osteoclastic action, causing an inhibition of bone resorption and consequently a suppression of bone remodeling and also antiangiogenic action, causing ischemia.2,5 Some theories, still under investigation, point out the effect of BP on MRONJ with inhibition of the immune system, susceptibility to infections and soft tissue toxicity by BP.2
The etiology of MRONJ is multifactorial2 as a result of the association of metabolic, local and genetic factors.7
Several studies have assessed risk factors for MRONJ.2,8–19 In the evaluation of the factors related to cancer treatment, MRONJ was associated with the type of BP,11,13,16 number of infusions,9,16 concomitance with corticosteroids13 and chemotherapy14 Some studies have shown an association between smoking9,19 and comorbidities such as diabetes and hypothyroidism9 with MRONJ. Evaluation of local factors showed the association of surgical dental procedures such as extraction and MRONJ.14
There are few studies that analyzed the association of salivary and plasma interleukin with the inflammatory process at the onset of MRONJ.20,21 Higher values in the salivary and blood levels of Interleukin-6 (IL-6) were observed in the group of MRONJ patients when compared to the group of patients treated with bisphosphonates, but without MRONJ, as well as to the control group, without the use of BP (p < 0.01), suggesting that L-6 could be used as a contributing marker in the diagnosis of MRONJ.20 Overproduction of IL-6 was related to autoimmune and inflammatory diseases.22,23
The aim of this study was to investigate the association of risk factors for MRONJ in cancer patients undergoing treatment with BP. Additionally, to evaluate salivary levels of IL-6 among patients ongoing or without BP and its association with MRONJ.
MethodsAccording to the Declaration of Helsinki for Human Studies, 1964, a case control study was conducted after approval by the Research Ethics Committee of the Institute of Cancer of São Paulo – Hospital 1 (Protocol nº 2,981,115) and Erasto Gaertner Cancer Center – Hospital 2 (Protocol nº 3,280,348) with the inclusion of seventy-four patients who signed the Informed Consent Form (ICF).
Patients with a diagnosis of breast cancer and other metastatic tumors for bone (prostate, kidney, lung, ovarian and uterus) and multiple myeloma were included in the study.
The patients were selected by convenience and divided into 3 groups according to clinical signs and oncological treatment. Group 1 was composed of 8 patients diagnosed with MRONJ on BP treatment, Group 2 with 60 patients on BP treatment, but without bone exposure and Group 3 with 6 patients prior to BP treatment. Patients included in Groups 1 and 2 were treated with zoledronic acid 4 mg at least 3 doses alone or after treatment with pamidronate 90 mg, with the last dose being used after up to 6 months from the salivary collection. The interval between infusion cycles varied from monthly to quarterly.
Patients with a history of radiotherapy or tumors (including metastases) involving the head and neck region were excluded from the study. In addition, patients undergoing prior surgical MRONJ treatment or diagnosis of autoimmune and inflammatory diseases such as rheumatoid arthritis, juvenile idiopathic arthritis syndrome and Castleman's disease were also excluded from the sample.
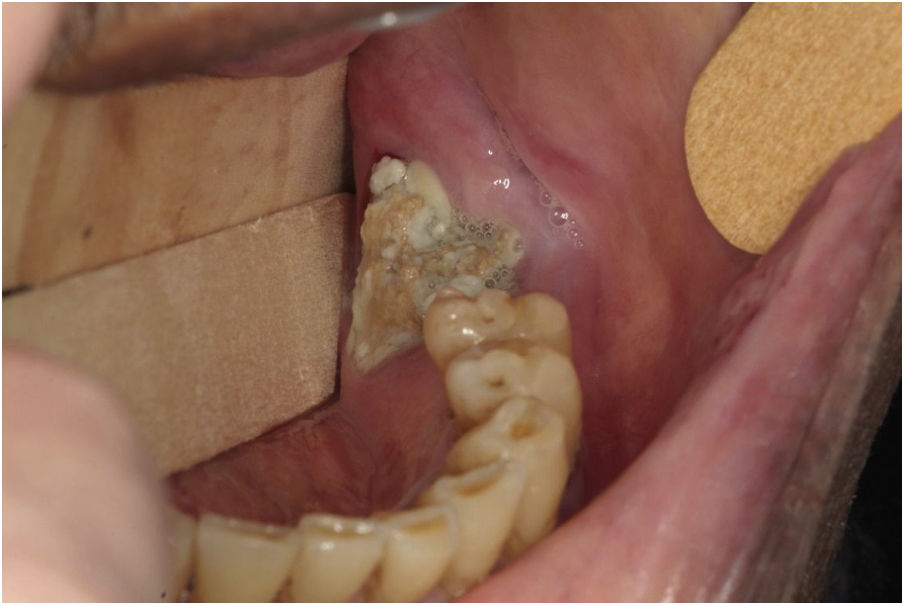
The MRONJ stages were classified as Stage 0: absence of clinical evidence of necrotic bone, presence of non-specific clinical findings with radiographic changes and symptomatology; Stage 1: exposed and necrotic bone or fistula, asymptomatic or absence of infection; Stage 2: presence of exposed and necrotic bone or fistula, infection evidenced by pain and erythema in the exposed bone region with or without purulent drainage; Stage 3: exposed and necrotic bone or fistula, infection and pain, exposed with necrotic bone extending below the alveolar bone region resulting in pathological fracture, extraoral fistula, buccal- sinus communication or osteolysis extending to lower mandibular border or floor sinus.4 Panoramic radiographic images were evaluated so that the correct diagnosis could be made, as well as the MRONJ classification. Fig. 1 shows example of MRONJ diagnosed after tooth extraction and classified as Stage 2.
Demographic information (gender, age, oncological/hematological diagnosis) and cancer treatment (type and number of BP infusions, chemotherapy and corticosteroid treatment concomitant with BP treatment) were collected from medical records.
Patients were evaluated regarding the performance of dental procedures during oncologic treatment such as infection outbreaks removal like periodontics, dentistry, endodontics and invasive dental procedures like extractions. Dental followup with specialized dentist care during BP treatment and smoking were also evaluated.
According to the technique described by Navazesh in 1993, the patients were instructed not to smoke, drink or eat at least 1 h before the dental appointment for the collection of the non-stimulated saliva. The patient was instructed to expectorate all saliva accumulated in the mouth within a 15 mL Falcon tube for 5 min without swallowing at intervals of 60 s.24 Saliva contaminated with blood was discarded. The saliva samples were immediately refrigerated and centrifuged at 3000 g for 10 min and stored at −80 °C until analysis.
The ELISA assay for human IL-6 was used for quantification of salivary IL-6 (Elabscience, Texas, USA), through colorimetric detection with a range between values of 7.81 and 500 pg/mL. Briefly, 100 u L of saliva were used into each well containing pre-coated human IL-6 specific antibody, incubation at 37 °C for 90 min. Biotinylated detection antibody specific for human IL-6 was then added and incubated at 37 °C for 1 h, followed by 3 washes. Avidin-Horseradish Peroxidase (HRP) was incubated at 37 °C for 1 h and washed 5 times. Finally, the substrate reagent was added, incubated at 37 °C for 15 min with the addition of stop solution. Measurement of optical density (OD) at wavelength 450 nm by spectrophotometry was evaluated and the calculation of human IL-6 concentration of the samples was performed by comparing the OD of the samples with the pre-established standard curve. Reactions were made in duplicate, according to the manufacturer's guidelines and compared among the 3 study groups.
The Statistical Package for the Social Sciences software (version 13, spss INC) was used for data analysis. A descriptive analysis of the data with frequency evaluation was performed. The Mann-Whitney test and the Kruskal–Wallis test were used to analyze the quantitative variables. The Kolmogorov-Smirnov test (n > 50) was used to analyze the data distribution normality. Fisher's test was used for the nominal categorical variables. Odds ratio was calculated to assess the chance of occurrence of a 95% Confidence Interval event (95% CI). The Spearman correlation coefficient was used for assessing the association of IL-6 levels with MRONJ severity. A p-value of less than 0.05 was considered statistically significant.
ResultsOf the 74 patients evaluated, 78.4% were women. The mean age in Groups 1, 2 and 3 were respectively 63.88, 56.27 and 51.33 years (p = 0.134). The oncologic diagnoses were breast cancer (n = 45), multiple myeloma (n = 20) and other solid tumors such as ovarian cancer, uterine cancer, kidney cancer, and lung cancer (n = 9). The demographic data of the participants were shown in Table 1.
Demographic distribution between Groups 1, 2 and 3.
| Group | Total | |||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Gender n (%) | ||||
| Female | 5 (62.5) | 48 (80.0) | 5 (83.3) | 58 |
| Male | 3 (37.5) | 12 (20.0) | 1 (16.7) | 16 |
| Mean age (standard deviation) | 63.8 (13.1) | 56.2 (11.9) | 51.3 (10.8) | |
| Diagnosis, n (%) | ||||
| Breast cancer | 5 (62.5) | 36 (60.0) | 4 (66.6) | 45 |
| Multiple myeloma | 2 (25.0) | 17 (28.3) | 1 (16.7) | 20 |
| Others | 1 (12.5) | 7 (11.7) | 1 (16.7) | 9 |
Group 1, patients with bisphosphonates and medication related osteonecrosis of the jaw; Group 2, patients with bisphosphonates and without medication related osteonecrosis of the jaw; Group 3, patients without bisphosphonates.
The median for salivary levels of IL-6 for Group 1 was 22.34 pg/mL (minimum 13.86 and maximum of 198.88); Group 2, 21.87 pg/mL (minimum of 10.45 and maximum of 75.91); and Group 3, 25.27 pg/mL (minimum 19.28 and maximum of 88.72). There was no statistically significant difference for the groups (p = 0.571). These results are shown in Table 2.
Salivary levels of IL-6 (pg/mL) between groups 1, 2 and 3.
| Group | p-Value | |||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Median IL-6 | 22.34 | 21.87 | 25.27 | 0.571a |
| Minimum–Maximum | (15.78–198.88) | (10.45–275.91) | (19.28–88.72) | |
Group 1, Patients with bisphosphonates and medication related osteonecrosis of the jaw; Group 2, Patients with bisphosphonates and without medication related osteonecrosis of the jaw; Group 3, Patients without bisphosphonates.
There was no association between salivary IL-6 levels and MRONJ stages (rs = −0.41 and p = 0.923).
The patients with MRONJ received a greater number of infusions of zoledronic acid 4 mg compared to patients without MRONJ using BP (p = 0.001). Among the patients who developed MRONJ, 7 patients (87.5%) were treated to at least 10 infusions.
Regarding the monthly or quarterly intervals of BP infusion, the monthly interval was associated with MRONJ (p = 0.044).
In the evaluation of cancer therapies concomitant with the use of BP in Group 1, chemotherapy treatment was present in 6 (75.0%) of 8 patients with MRONJ (p = 1.000) and corticosteroid treatment was present in 7 (87.5%) of the 8 patients with MRONJ (p = 0.427).
Table 3 presents the data of the oncological treatment as the type and number of BP infusions, interval between infusions, concomitant chemotherapy and corticosteroids use with BP (Groups 1 and 2).
Assessment of systemic and local risk factors for MRONJ between groups 1 and 2.
| Group | p-Value | OR | 95% IC | ||
|---|---|---|---|---|---|
| 1 | 2 | ||||
| BP type, n (%) | |||||
| Zoledronic acid | 7 (87.5) | 53 (88.3) | 1.000a | 0.92 | 0.099–8.673 |
| Pamidronate and zoledronic acid | 1 (12.5) | 7 (11.8) | |||
| Number of cycles zoledronic acid | |||||
| Median (Minimum–Maximum) | 16.0 (10–32) | 7.0 (3–38) | 0.001b | ||
| Number of cycles Pamidronate | |||||
| Median (Minimum–Maximum) | 0.00 (0–25) | 0.00 (0–18) | 0.852b | ||
| Infusion interval, n (%) | |||||
| Monthly | 6 (75.0) | 19 (31.7) | 0.044a | 7.75 | 1.194–35.092 |
| Quartely | 2 (25.0) | 41 (68.2) | |||
| Chemotherapy, n (%) | |||||
| Yes | 6 (75.0) | 43 (70.0) | 1.000a | 1.18 | 0.155–4.597 |
| No | 2 (25.0) | 17 (28.3) | |||
| Steroids, n (%) | |||||
| Yes | 7 (87.5) | 42 (70.0) | 0.427a | 3.20 | 0.038–2.910 |
| No | 1 (12.5) | 18 (30.0) | |||
| Check-up and procedures trans BP, n (%) | |||||
| No | 6 (75.0) | 18 (30.0) | 0.019a | 8.25 | 1.288–38.046 |
| Yes | 2 (25.0) | 42 (70.0) | |||
| Exodontia, n (%) | |||||
| Yes | 6 (75.0) | 25 (41.7) | 0.129a | 4.80 | 0.044–1.278 |
| No | 2 (25.0) | 35 (58.3) | |||
| Smoking, n (%) | |||||
| Yes (ex-smoking < 5 years) | 3 (37.5) | 4 (6.7) | 0.031a | 9.37 | 1.453–48.549 |
| No (ex-smoking > 5 years) | 5 (62.5) | 56 (93.3) | |||
Group 1, patients with bisphosphonates and medication related osteonecrosis of the jaw; Group 2, patients with bisphosphonates and without medication related osteonecrosis of the jaw.
Patients without a dental check-up routine with procedures for eliminating infection outbreaks (caries lesions, periodontal disease, endodontics and exodontia) or prosthetic traumas had association with MRONJ (p = 0.019).
Of the dental risk factors for MRONJ, 26 patients (38,2%) out of 68 trans- BP patients were submitted to exodontias (Hospital 1). There was no significant difference between the Groups 1 and 2 to exodontias (p = 0.129). However, of the 8 patients who developed MRONJ, 6 patients (75.0%) had occurrence after surgical dental procedures and 2 cases occurred spontaneously (25.0%). Of the 6 patients who developed MRONJ after surgical procedures, in 5 cases the procedures were performed in external services and referral for MRONJ treatment for Hospital 1 (1 patient) and Hospital 2 (4 patients). Only 1 patient with MRONJ (Stage 1) occurred after extraction at Hospital 1.
The maxilla was the site of MRONJ's greatest involvement. Four patients (50.0%) developed MRONJ in the maxilla, 3 patients (37.5%) in the mandible, while 1 (12.5%) presented simultaneous bone exposure in the mandible and posterior maxilla.
Smoking was a factor associated with MRONJ (p = 0.031). The odds ratio for smoking was 9.37.
Table 3 presents data on dental followup during treatment with BP, tooth extraction and smoking in Groups 1 and 2.
Fig. 1 illustrates the case of patient number 6, with MRONJ Stage 2, after exodontia of 38 tooth during antiresorptive therapy.
Table 4 summarizes the demographic data, BP treatment and clinical data from 8 patients in Group 1.
Demographic, clinical and cancer treatment characteristics of Group 1.
| Patient (Nº/age/gender) | Diagnosis | Type of BP | Number of infusions | MRONJ Stage (AAOMS 2014) | Location | Factor MRONJ trigger | Painful symptoms |
|---|---|---|---|---|---|---|---|
| 1/65/F | Breast | AZ | 13 | 2 | Anterior Mx | Implant | Yes |
| 2/77/F | Breast | AZ | 22 | 1 | Posterior Mx | Exodontia | No |
| 3/58/F | Breast | AZ | 33 | 0 | Posterior Md | Spontaneous | No |
| 4/47/F | Breast | AZ | 10 | 2 | Posterior Mx | Exodontia | Yes |
| 5/64/M | MM | AZ | 13 | 2 | Anterior Mx | Exodontia | Yes |
| 6/88/M | Prostate | AZ | 11 | 2 | Posterior Md | Exodontia | Yes |
| 7/58/F | Breast | AZ | 33 | 1 | Posterior Md | Spontaneous | No |
| 8/54/F | MM | AZ | 19 | 2 | Posterior Mx | Exodontia | Yes |
| P | 25 | Posterior Md |
P, bisphosphonate; AZ, zolendronic acid; P, pamidronate; F, female; M, male; MM, multiple myeloma; MRONJ, medication related osteonecrosis of the jaw; Md, mandibula; Mx, maxilla.
The incidence of MRONJ among cancer patients on intravenous BP may vary from 1.2% to 9.9%.2 This incidence is higher among patients with multiple myeloma compared to patients with breast or prostate cancer.5,9,16 Our study, in turn, presented the frequency of 10.8% in the sample of patients studied, a figure higher than that reported in the literature, probably due the fact that the study was conducted at the two cancer treatment centers with a specialized Service of Maxillofacial Surgery, which makes them a reference center for the treatment of MRONJ.
Most of MRONJ cases were diagnosed with breast cancer, while 1 had prostate cancer and 2 patients had multiple myeloma (MM). Our sample had a higher number of breast cancer, the most frequent in our region, and BP infusion protocols, such as dose and periodicity for solid tumors with osseous metastases and for MM do not differ in the sample of patients studied.
Several studies have shown time of exposure to BP as a risk factor for the development of MRONJ8,9,14,16 and have also shown an association between the number of BP infusions and MRONJ development.9,16 In studies evaluating BP exposure time, infusion protocols ranged from 3 to 4 weeks, and were associated with the development of MRONJ. In our study, it was not feasible to assess the time of exposure MRONJ risk, since the patients included in the study had interval protocols between BP infusions ranging monthly and quarterly. In our study, we evaluated the number of infusions and the group of patients with MRONJ who received a higher number of 4 mg zoledronic acid infusions compared to the group of patients without MRONJ (p = 0.001). Our results are in agreement with the studies that reported an association of the number of infusions with MRONJ.8,9,16
Some studies have shown that the risk for MRONJ was significantly higher in patients taking zoledronic acid compared with pamidronate alone or after pamidronate.9,16,25 This fact explains that the potency of zoledronic acid is 100 times greater than pamidronate.5 Our study showed that MRONJ was associated with a higher number of zoledronic acid cycles (p = 0.001) as described in the literature.
Regarding the BP infusion interval, there is evidence that quarterly infusions may decrease the risk for MRONJ compared to monthly infusions. A study has shown that risk for MRONJ decreases 8-fold in quarterly infusions compared with monthly infusions (p = 0.049).25 Another study showed that the quarterly regimen was not inferior to the monthly regimen for effectiveness of cancer treatment and 2 cases of MRONJ were associated with the monthly infusion group.26 Our study showed association of MRONJ with monthly infusion interval (p = 0.044), in agreement with the literature. Patients with monthly BP infusion interval have 7.75 times more chance for MRONJ compared to those with quarterly infusion.
The association of BP use concomitant with chemotherapeutic has been previously reported.14 However, the study did not classify the types of chemotherapy used according to the type of cancer diagnosis to assess the risk for MRONJ.14 In our study, 6 patients (75.0%) of the 8 MRONJ patients had chemotherapy treatment concomitant with BP treatment. However, we have to consider the diversity of chemotherapy regimens used due to the different tumor diagnoses in the sample, which compromises the evaluation of concomitant chemotherapy with BP in the development of MRONJ.
For some authors, MRONJ is associated with the concomitant treatment of corticosteroids with antiresorptive agents.6,18,27 The immunosuppressive effect of corticosteroids may be related to delayed healing, alteration of the oral microbiota and higher risk for infection and development of MRONJ.18 Study has shown an increased risk of MRONJ as corticosteroid use, but did not classify the risk according to clinical indication and corticosteroid dose.13 In our study, MRONJ patients presented an 87.5% frequency of corticosteroids use compared with 12.5% of patients without corticosteroids with MRONJ (p = 0.427). As previously mentioned for the chemotherapeutic treatment, the different protocols and periodicity of corticosteroids in the concomitant treatment with BP may make it unfeasible to use this data to predict the risk for MRONJ.
Regarding the dental factors that trigger MRONJ, we can mention trauma, spontaneous cause,28 presence of pre-existing dental diseases, such as periodontitis and surgical dental procedures.8 However, tooth extraction is the local factor predominantly associated with MRONJ.18 Several studies have shown the association of MRONJ with dental extraction.9,15,16,25 Conscious of what has been exposed, it is thought that non-surgical dental procedures can prevent MRONJ8 In our study, of the 8 patients who developed MRONJ, 6 cases of MRONJ occurred after surgical procedures and 2 cases were spontaneous (p = 0.129). In 5 cases of MRONJ, the surgical procedures were performed in external services and referral for MRONJ treatment in hospitals 1 (1 case) and 2 (4 cases). This result shows the importance of performing dental extraction by skilled professionals, as shown in 26 cases of trans BP extractions performed at Hospital 1, and just 1 case of MRONJ (Stage 1). Hospital 1 performed a minimally traumatic surgical protocol, primary closure of the surgical area, pre- and postoperative antibiotic therapy with amoxicillin 500 mg or clindamycin 600 mg for patients allergic to penicillin and periodic followup with these patients. These measures may decrease the risk for MRONJ and thus justify the absence of association of extraction with MRONJ in this study.
MRONJ lesions appear more commonly in the mandible than in the maxilla, in the 2:1 ratio. However, it may develop in both arches.6 The reason for MRONJ predilection in the mandible when compared to the maxilla may be due to the high mandibular bone remodeling suppressed by antiresorptive agents14 and also structurally by the thick bone cortical and medullary cavity.6
Some studies have shown that the mandible is the most affected site for MRONJ and, most of the time, associated with exodontia2,7,8,10,12,14,16,27 In our study, however, the region of greatest involvement with MRONJ was the maxilla, contrary to what is reported in previous reports. This fact can be explained because 4 of the 8 cases were associated with surgical dental procedures exclusively in the maxilla. In the cases of MRONJ of spontaneous cause, the mandible was affected, consistent with that reported in the literature.
Pre-BP dental therapy with the institution of preventive measures of dental care in routine visits to the dental surgeon3,27,29,30 has had a direct implication in reducing the incidence of MRONJ.2,8,18 Thus, invasive dental treatment, such as extractions, should be performed prior to BP therapy, in order to reduce the need for these procedures trans BP.8,10,12,29 During BP therapy, however, clinical and radiographic dental monitoring of these patients is also important.8 Otto et al. 2012, showed that only 7 (10.6%) of 66 patients with MRONJ were referred to the dentist before BP treatment.2 Water et al. 2008, reported that 6 out of 8 MRONJ patients (75.0%) had routine dental appointments.15 The sample of this study originated from services with different care protocols, while Hospital 1 is characterized as a clinical dentistry service performing procedures such as dentistry, endodontics, periodontics and extractions; also radiographic/clinical followup occurred up to 1 year after last BP infusion, Hospital 2 is characterized by an oral maxillofacial surgery service that provides guidance and prevention, but with limitations in performing clinical procedures, referring patients when necessary. The importance of trans-BP dental monitoring is highlighted as a result of our study, since 6 patients (75.0%) with MRONJ did not have trans BP dental checkup with dental procedures (dentistry, endodontics, periodontics and extractions) and only 2 patients (25.5%) had routine appointments for dental procedures. This result is a statistical difference between Groups 1 and 2 (p = 0.019). Our results showed that patients who did not eliminate foci of infection before or during BP therapy showed a higher risk for MRONJ, according to Otto et al. 2012.2 It is believed that patients undergoing trans-BP dental checkups have hygiene orientation, procedures such as removal of infection outbreaks and preventive procedures and these measures will have a direct impact on the development of MRONJ.
Other studies have reported smoking as a risk factor for MRONJ9,19 The result of our study showed that smokers are 9.37 times more likely to develop MRONJ than those without the habit (p = 0.031). Smoking promotes delayed bone healing and worsening of the periodontal condition.31,32 Among the deleterious effects of toxins found in tobacco smoke are vasoconstriction and platelet aggregation induced by nicotine and reduced oxygen transport capacity by hemoglobin.31 Increased vasoconstriction may lead to ischemia that is associated with MRONJ pathophysiology.
In bone metabolism, IL-6 induces osteoclast differentiation, resulting in bone resorption.22 BPs affect osteoblast production through osteoclastogenesis mediators, including Kappa B Nuclear Activation receptor Ligant (RANKL), Osteoprotegerin (OPG), and IL-6, which results in decreased formation/activation of osteoclasts. This event promotes an increase in the production of IL-6 and OPG and a decrease in the production of RANKL, inhibiting bone turnover and causing bone necrosis.32 The mucosal evaluation of MRONJ patients also showed significantly elevated levels of IL-6 levels and RANKL/OPG ratio in patients with MRONJ, suggesting the importance of evaluating these markers during BP therapy to monitor the onset of MRONJ.28 In the infectious process during bone necrosis, inflammatory response mediators, IL-6 and tumor necrosis factor alpha (TNF-alpha) showed moderately high levels in MRONJ cases.33 Studies showed elevated salivary levels of IL-1 alpha, IL-1RA and IL-1 beta in inflammatory processes of MRONJ.21 Another study reported significantly higher values for plasma and saliva IL-6 in the MRONJ patient group compared with control groups.20 In the present study, no association was found between salivary concentration of IL-6 with MRONJ (p = 0.571). The median of IL-6 was higher in Group 3 patients, who did not receive chemotherapy treatment, and did not present oral adequacy and therefore high levels of IL-6 could be associated with periodontal condition. Another hypothesis for higher levels of IL-6 in Group 3 patients may be associated with the therapeutic factor, not yet started, and that depending on the prescribed chemotherapeutic agent may decrease IL-6 levels.23 There was no association between higher stages of MRONJ and higher salivary IL-6 levels (p = 0.923), as previously reported.20
ConclusionRisk factors, such as a higher number of cycles of zoledronic acid, monthly infusion protocol, no trans BP dental checkups with dental procedures, including extractions and smoking were associated with higher risk of development of MRONJ. Other factors, such as concomitant treatment with corticosteroids and chemotherapeutic agents, and invasive dental procedures, were more frequent in patients with MRONJ. A salivary IL-6 level was not associated with higher risk and severity for MRONJ. Dental followup by a specialized team with procedures to remove foci of infection during treatment with BPs showed an important aspect in preventing this complication.
Surgical dental procedures are inevitable in some cases during treatment with BP and when performed by specialized oral surgeons can improve patient outcome and reduce the risk to develop MRONJ.
Conflicts of interestThe authors declare no conflicts of interest.
CAPES - Coordination of Higher Education Improvement.
Peer Review under the responsibility of Associação Brasileira de Otorrinolaringologia e Cirurgia Cérvico-Facial.