The aim of this study was to assess the relationship between the stimulation amplitude and the distance to the facial nerve.
MethodsThis study was designed as a prospective clinical study. A total of 20 patients (12 males, 8 females) were included. Partial superficial parotidectomy was performed in all patients with intraoperative facial monitoring. Measurements were made on the main trunk and major branches. Stimulation was started at 1 mA and incrementally increased to 2 and 3 mA's. The shortest distance creating a robust response (>100 mV) was recorded.
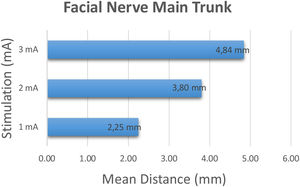
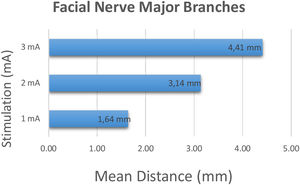
ResultsAt 1 mA, 2 mA and 3 mA stimulation intensity, the average distance between the tip of the stimulation probe and the main trunk was 2.20 ± 0.76 mm (range 1–3 mm), 3.80 ± 0.95 mm (range 2–5 mm), 4.80 ± 1.05 mm (range 3–7 mm) respectively. The stimulus intensity was inversely proportional in respect to the distance between the nerve and the tip of the stimulus probe (P < .00). The same relation was present in the facial nerve major branch measurements (P < .00).
ConclusionThe proportional stimulation amplitude and distance to the facial nerve is thought to be a reliable auxillary method to assist the surgeon by facilitating the estimation of the distance to the facial nerve during extracapsular dissection and minimally invasive cases where the facial nerve isn’t routinely dissected.
Level of evidenceLevel 3.
Surgery is the standard treatment for salivary gland neoplasms. Different authors have proposed classifications for parotid surgeries. For example, Snow proposed to consider five types of surgery: superficial parotidectomy, total parotidectomy (those two were called formal parotidectomies), partial superficial parotidectomy, selective deep lobe parotidectomy (he calls them partial parotidectomies), and finally extracapsular dissection.1 Parotid gland surgery has always been a challenging surgery due to its close relationship with the facial nerve. One of the most feared complications for both the patient and the surgeon is facial nerve palsy. Facial nerve palsy can cause cosmetic and functional morbidity by affecting all motor functions of the ipsilateral face, causing ocular complications, affecting eating and drinking functions, and consequently, can seriously affect the quality of life.2 Therefore, preserving the anatomic and functional integrity of the facial nerve during parotid gland surgery is very important to reduce postoperative morbidity. The reported incidence of facial weakness immediately after parotid tumor surgery ranges from 14% to 65%.3 Also, a much higher rate of facial nerve paresis is seen in revision parotid surgery.3,4
Direct observation and surgical preservation of the facial nerve is the gold standard method, while facial nerve monitoring is the most commonly used auxiliary technique in the current medical practice. About 75% of the otolaryngologists in Germany and over 67%–80% in the United Kingdom use nerve monitoring during parotid surgery.5 According to another study conducted in the United States, while the use of facial nerve monitoring during parotidectomy is 60%, this rate is 79% in surgeons who perform more than 10 parotidectomies per year.6
The purpose of facial nerve monitoring is to provide early detection-recognition of the facial nerve and its branches, early detection of potentially harmful manipulations, enable the tracking and course of the nerve, reduce mechanical trauma, and provide prognostic information about postoperative nerve function.7
The aim of this study was to predict the distance to the facial nerve during dissection using the facial nerve stimulator.
MethodsThis study was performed at a tertiary academic center between July 2020 and January 2021. All procedures performed in studies involving human participants were in concordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study before control visits. Institutional review board approval was obtained (IRB approval n° 19-10T-24).
Patient selection and data collectionPartial superficial parotidectomy was performed with facial monitoring in all patients. Among the total of 38 patients who underwent partial superficial parotidectomy procedures between July 2020 and January 2021, a total of 20 patients (12 males, 8 females ) that met the inclusion criteria were included. The inclusion criteria were: pathologically proven benign FNAB, lack of deep lobe involvement, and tumor diameter less than 4 cm in the preoperative radiological evaluation. Patients with suspicious malignant lesions, recurrent cases, and previous history of facial paralysis were excluded.
All demograhpic, laboratory and imaging data were collected from electronic health records. Intraoperative findings and measurements were collected and recorded on computer by the surgeons. All data regarding this study is secured, backed-up, and only accessible by the authors of this study.
Instrumentation and procedureThe study was designed as a prospective study. All data were collected prospectively. These included demographic data, results of Fine-Needle Aspiration Biopsy (FNAB), the stimulation distance created by stimulating the facial nerve trunk, the stimulation distance created by stimulating the facial nerve main branches, postoperative pathology results, preoperative and postoperative facial nerve examination.
Partial superficial parotidectomy was performed in all patients with intraoperative facial monitoring. During the surgery, only a short-acting (rocuronium) muscle relaxant was administered during the induction phase of anesthesia. A 4-channel NIM-Response® 3.0 neuromonitoring device was used for neuromonitoring (Medtronic plc, Dublin, Ireland). Neuromonitoring devices have an impulse-generating stimulator to generate electrically evoked EMG potentials. This enables active monitoring.
The first stage of neuromonitoring was the placement of electrodes. Needle electrodes were positioned subdermally at the most appropriate place to measure the activity of facial muscles. These needle electrodes were placed in the orbicularis oculi muscle (temporal and zygomatic nerve), orbicularis oris muscle (buccal nerve), in a 2-channel system. In addition to these electrodes, the grounding and anode electrodes of the stimulator were also placed, the stimulator was placed in the surgical field and all electrodes were connected to the detector part.
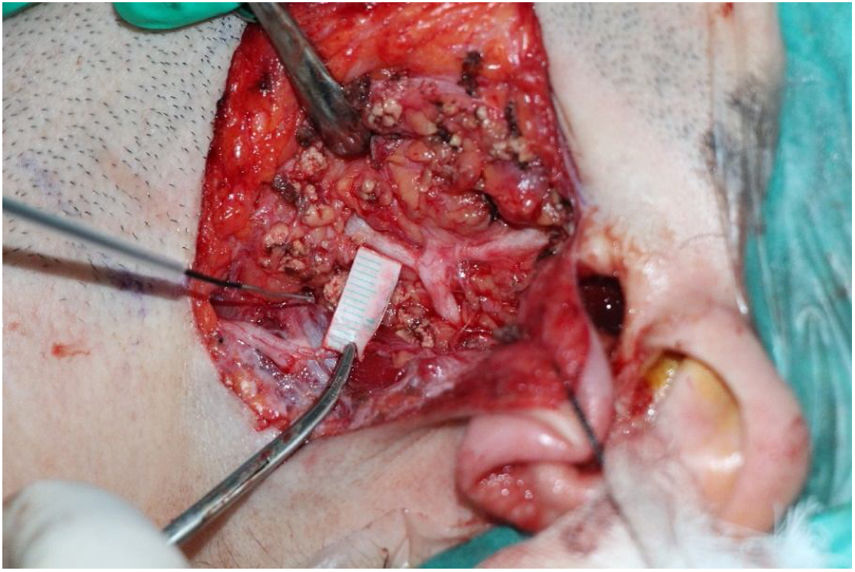
The surgery starts with modified Blair incision. The facial nerve main trunk was identified using landmarks. The major branches and distal branches were dissected according to the localization and size of the tumor. Measurement were performed after partial superficial parotidectomy was done. We made the measurements on the main trunk in all patients and on major branches if identified. An individualized ruler of 10 mm size was prepared from the surgical ruler. The ruler was placed perpendicular to the nerve on the remaining parotid gland tissue and the stimulation started from the periphery of the nerve until the shortest distance creating a robust (>100 mV) response was recorded (Fig. 1). Great care was given to stimulate the nerve from the remaining parotid gland tissue, on a plane parallel to the facial nerve and it’s braches. Stimulation was started at 1 mA and incrementally increased to 2 and 3 mA's. The main trunk was measured in all patients, if the upper or lower main branches were identified they were measured, too.
Statistics analysisStatistical analysis was done using computer software SPSVR version 25.0 (SPSS Inc. Chicago, IL). Descriptive statistics were used for analysing demographic data and preoperative and postoperative patology results. Parametric test statics were used Anova test. The Shapiro-Wilk test was used for determining the distribution pattern of the data. According to the Shapiro-Wilk test statistics (P < .05), non-parametric test statistics were used because the variables were not suitable for normal distribution. Kruskal Wallis test used to compare three groups.
ResultsThe mean age of the patients was 45.18 ± 11.24 years (range 20–79, 8 females, 12 males). FNAB results were pleomorphic adenoma in 11 (55%) of the 20 patients, whartin’s tumor in 5 (25%) patient, and benign cytology in 4 (20%) patients. Postoperative pathology results of was pleomorphic adenoma in 13 (65%) patients, whartin's tumor in 5 (25%) patients, chronic sialoadenitis in 1 (5%) patient, and myoepithelioma in 1 (5%) patient. The mean tumor diameter according to the postoperative pathology specimens was 2.4 ± 0.9 cm (min 1, max 6.5 cm) in patients. Most comon localization was the parotid tail (16 patients ‒ 80%) followed by the preauricular region (4 patients ‒ 20%).
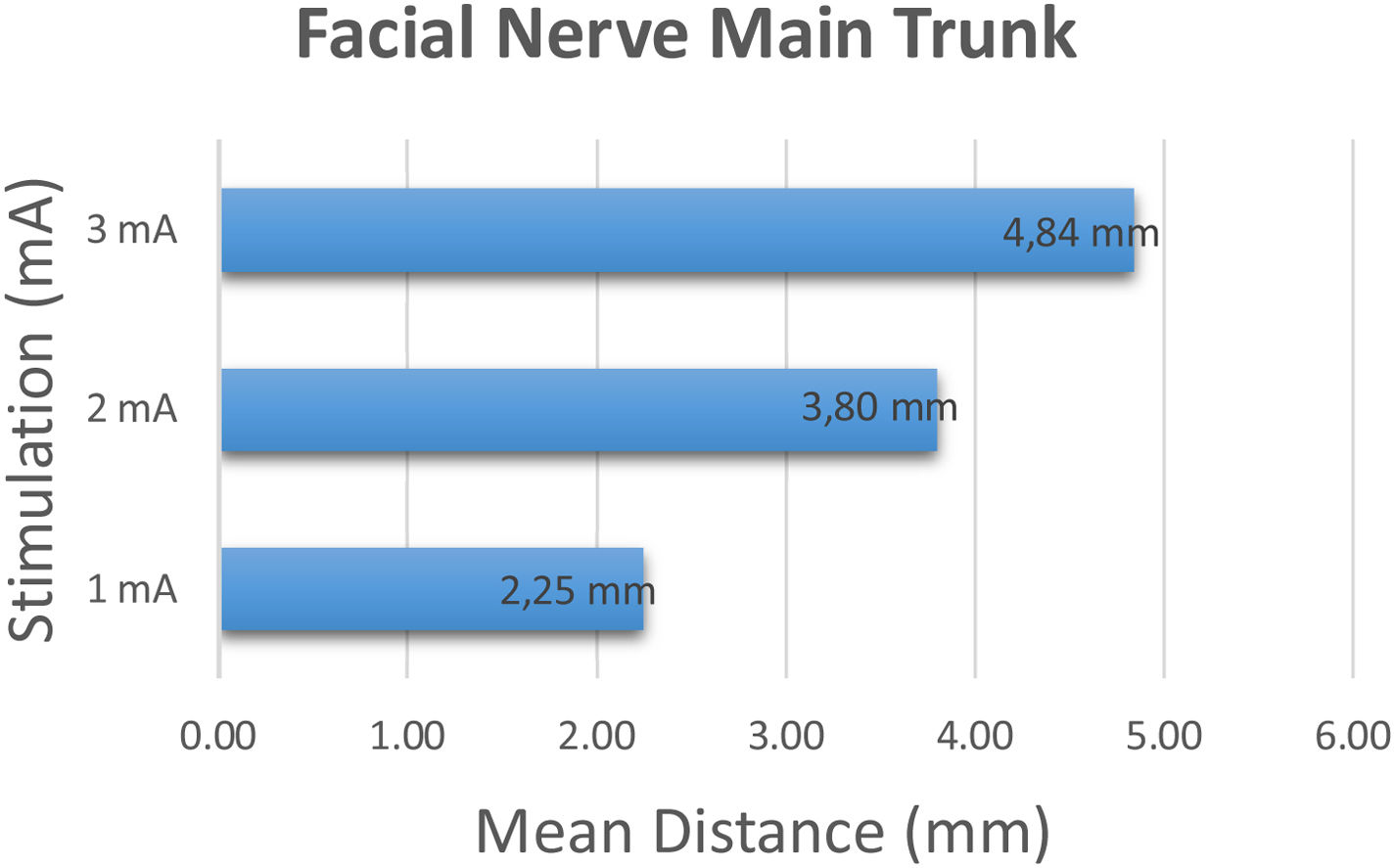
The facial nerve main trunk was identified and stimulation was started at 1 mA and incrementally increased to 2 and 3 mA's. The shortest distance creating a robust (>100 mV) response was recorded. At 1 mA stimulation intensity, the average distance between the tip of the stimulation probe and the main trunk was 2.20 ± 0.76 mm (range 1–3 mm). At 2 mA and 3 mA stimulation intensity, the average distance between the tip of the stimulation probe and the main trunk was 3.80 ± 0.95 mm (range 2–5 mm), 4.80 ± 1.05 mm (range 3–7 mm) respectively (Fig. 2).
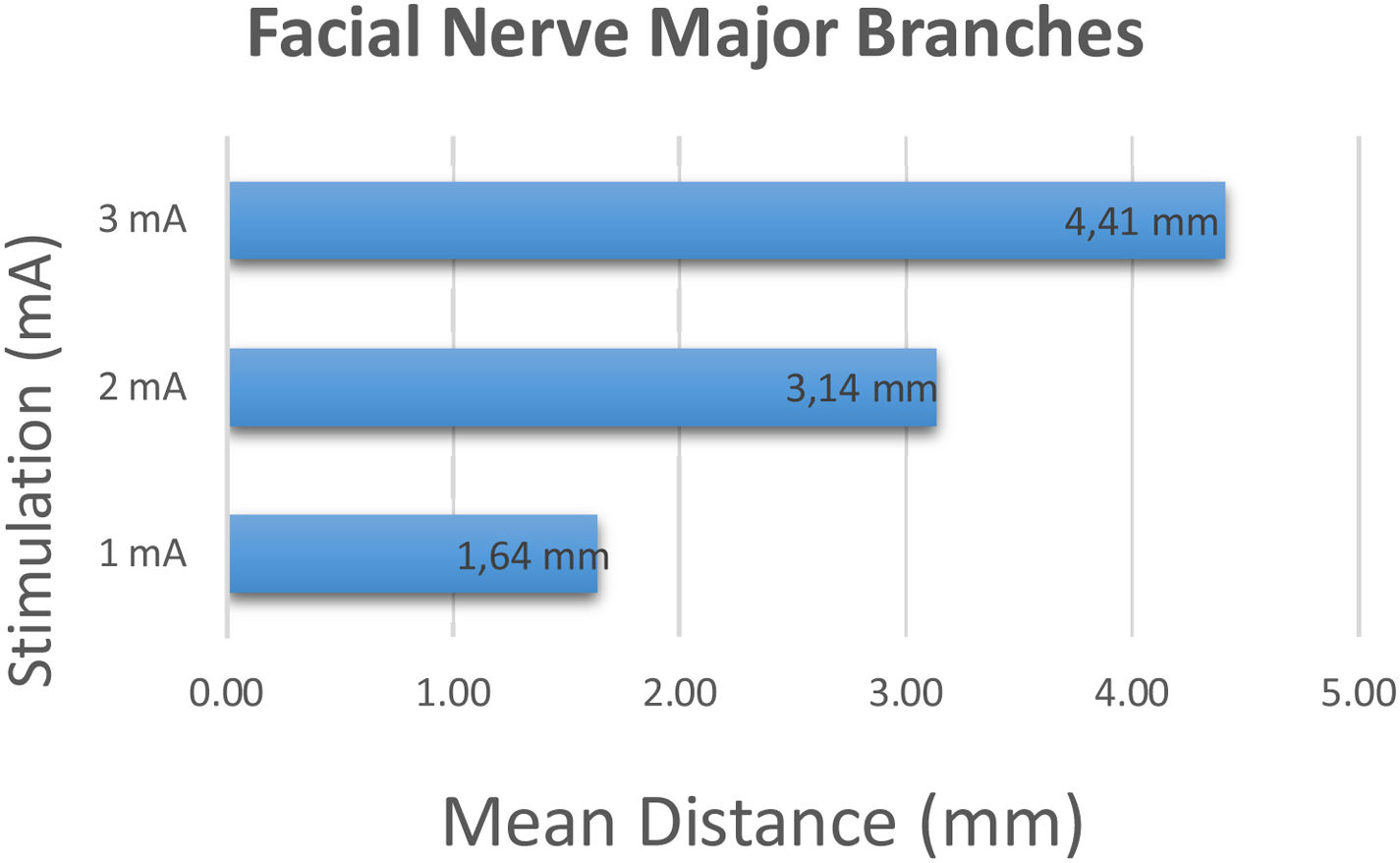
Major branches of 22 facial nerves including 16 lower branches and 6 upper branches were identified perioperatively and stimulation was started at 1 mA and incrementally increased to 2 and 3 mA's. At 1 mA stimulation intensity, the average distance between the tip of the stimulation probe and the major branches was 1.64 ± 0.95 mm (range 1–5 mm). At 2 mA and 3 mA stimulation intensity, the average distance between the place where the stimulation first occurred and the major branch was 3.14 ± 1.75 mm (range 1–9 mm), 4.41 ± 2.06 mm (range 2–10 mm) respectively (Fig. 3).
Regarding the facial nerve main trunk the stimulus intensity was inversely proportional in respect to the distance between the nerve and the tip of the stimulus probe (P < .00). The same relation was present in the facial nerve major branch measurements (P < .00).
The mean shortest distance in 1 mA and 2 mA stimulations were significantly longer for main trunk than major branches (P < .05). However, in 3 mA stimulation, there were no significant difference of the mean shortest distance between main trunk and major branches (P > .05) (Table 1).
Mean distance of stimulation thresholds with facial nerve main trunk and major branches.
| 1 mA | 2 mA | 3 mA | |
|---|---|---|---|
| Mean distance (mm) | Mean distance (mm) | Mean distance (mm) | |
| Main trunk | 2.20 ± 0.76 mm | 3.80 ± 0.95 mm | 4.80 ± 1.05 mm |
| Major branches | 1.64 ± 0.95 mm | 3.14 ± 1.75 mm | 4.41 ± 2.06 mm |
| P-value | .003 | .023 | .28 |
The purpose of facial nerve monitoring is to provide early detection-recognition of the facial nerve and its branches, early detection of potentially harmful manipulations, enable the tracking and course of the nerve, reduce mechanical trauma, and provide prognostic information about postoperative nerve function.5–9 Although facial nerve monitoring provides many advantages, controversy continues over its routine use.6–10 Lowry et al. ın a study involving 3199 otolaryngologists, the most common reasons for using intraoperative monitoring were found to be the presence of the nerve (20%), medicolegal concerns (14%), safety (11%), and the belief that monitoring is standard practice (11%).6 Routine use of facial nerve monitoring is recommended in large, bulky tumors and revision surgeries where nerve dissection and direct detection are difficult.11 Witt et al. reported that monitoring reduced surgical time and decreased the incidence of postoperative facial paralysis in recurrent pleomorphic adenomas undergoing total parotidectomy and wide resection, but its effect was limited in superficial parotidectomy.12 Facial nerve monitoring is the standart of care in all types of parotid surgery at our instutation. Liu et al. reported that facial nerve monitoring did not affect temporary and permanent postoperative facial paralysis in patients with recurrent pleomorphic adenoma surgery, but significantly reduced surgical time in wide excision or total parotidectomy.13 Routine use of monitoring is recommended in patients who have undergone radiotherapy, malignant masses, chronic parotitis, and minimally invasive procedures such as intraparotid sentinel lymph node biopsy and extracapsular dissection.14,15
Some authors argue that over-reliance on facial nerve monitoring will create a false sense of confidence in the surgeon and reduce the sensitivity of surgical dissection.6,12 In a recent meta-analysis involving 546 patients, it was reported that intraoperative facial nerve monitoring significantly reduced early postoperative facial nerve weakness (22.5% vs. 34.2%), but did not significantly increase postoperative permanent facial paralysis (3.9% vs. 7.1%) compared to the control group.10 Therefore, the authors recommend the routine use of intraoperative facial nerve monitoring, although it does not significantly affect the final results, especially in today's world, where the expectations of patients and quality standards in healthcare services are high.10
Intraoperative stimulation thresholds and amplitudes can also provide some information about postoperative nerve prognosis. Mamelle et al. reported that stimulation at the supramaximal level (2 mA) might be useful in predicting facial nerve damage in the early postoperative period.16
The surgeon's knowledge of anatomy and experience becomes more important in less invasive surgeries such as partial superficial parotidectomy and extracapsular dissection where the facial nerve and the branches are not routinely dissected or a limited nerve dissection is performed. Aside from the surgical skill we wanted to know how close we were to the facial nerve with distant stimulation. We think our results provided reliable information to the surgeon. According to the measuretments the distance to the facial nerve was at least 1.5 mm when the stimulus intensity was set to 1 mA, 3 mm at 2 mA, and 4 mm at 3 mA. The nerve was significantly stimulated from a distant point as the stimulus intensity was gradually increased. Considering this stimulus and distance relationship, this might guide the surgeon in less invasive surgical procedures in which the facial nerve is not surgically dissected.
The main limitation of this study is the small number of patients. Elective cases had to be cancelled multiple times due to the pikes in COVİD-19 cases. More dissection is performed around the facial nerve main trunk compared to the smaller braches. This could have affected the results by two mechanisms: 1) Dissection trauma related to the surgical instruments; 2) Tissue loss around the nerve might have altered the electrical current of the nerve stimulator. However, we think that the distance-amplitude relationship is an important marker in finding the facial nerve, provides reliable information and can be used as a navigation tool. Additionally the surgeon has to be very careful to stimute the nerve from the parotid gland tissue because other tissues such as fat or muscle tissues may have different electric potential gradients. The relationship between distance and stimulation levels can be used in future research and may be a standard measurement technique used in parotid surgery.
ConclusionThe proportional stimulation amplitude and distance to the facial nerve is thought to be reliable auxillary method to assist the surgeon by facilitating the estimation of the distance to the facial nerve during extracapsular dissection and minimally invasive cases where the facial nerve is not routinely identified and dissected. However, it should be kept in mind that this can not replace surgeon experience and thorough anatomical knowledge.
Conflicts of interestThe authors declare no conflicts of interest.
This study was supported by the Ege University Scientific Research Projects Department (Project ID: TGA-2020-21093).
Peer Review under the responsibility of Associação Brasileira de Otorrinolaringologia e Cirurgia Cérvico-Facial.