To describe the process of translation into Brazilian Portuguese and cross-cultural adaptation of the French Reflux Symptom Score-12 questionnaire used for the diagnosis of laryngopharyngeal reflux.
MethodsThis was a cross-cultural translation and adaptation study of a health instrument, with a cross-sectional design. It was carried out in eight stages: translation from French into Brazilian Portuguese, cultural adaptation by a panel of experts, application of the first version (pilot test 1), adaptation by a panel of experts, application of the second version (pilot test 2), back translation, reviewing by a committee in conjunction with the author of the original instrument and, application of the final version. The Brazilian Portuguese versions of the questionnaire were applied to individuals with symptoms and signs of laryngopharyngeal reflux who underwent pHmetry and esophageal manometry at the study site.
ResultsIn pilot test 1, the first version of the RSS-12 in Brazilian Portuguese was applied to 30 patients. The patients had no difficulty to understand any of the 12 symptom items, but 15 patients (50%) had difficulty interpreting the symptom frequency score. After adapting the format of the frequency score, a version 2 of the RSS-12 in Brazilian Portuguese was applied to another 23 patients, who completed the questionnaire in full without any difficulty. Along with the review committee, the author of the original RSS-12 considered the version 2 to be adequate and did not propose any changes, so it was approved as the final version of the Brazilian Portuguese RSS-12.
ConclusionThe Brazilian Portuguese version of the instrument, called Reflux Symptom Score-12 PT-BR, shows good understanding and linguistic, conceptual and content equivalence, in relation to the original Reflux Symptom Score-12.
Laryngopharyngeal reflux (LPR) is an inflammatory condition of the upper aerodigestive tract related to direct and indirect effects of reflux of the gastroduodenal contents. It is a frequent condition, accounting for 10% of the otorhinolaryngology (ORL) visits.1,2 The most prevalent symptoms include pharyngeal globus, hoarseness, cough, throat clearing, and postnasal drip.3 At the laryngoscopy, the most common found signs are erythema of the arytenoids, interarytenoid region, and thick endolaryngeal mucus.4,5 In less than 50% of the cases, these complaints are associated with symptoms attributed to gastroesophageal reflux disease (GERD), such as heartburn and regurgitation.6 The clinical picture can resemble many ORL conditions, such as allergy, rhinosinusitis, and chronic laryngitis related to tobacco or alcohol consumption.3 Patients with LPR have impaired quality of life and require prolonged drug treatment, which is associated with significant costs and changes in behavior and dietary habits.7,8
The diagnosis of LPR can be challenging since the symptomatology is nonspecific and so far there is no gold standard diagnostic test.9,10 The most accurate complementary method is the dual channel impedance-pHmetry (MII)/pH-metry, considered to be an invasive, expensive, and scarcely available test.11,12 In patients with suggestive symptoms and signs, one episode of proximal esophageal reflux seen in the MII-pH examination, either acidic or non-acidic, supports the diagnosis of LPR.3,12
The use of Patient-Related Outcome Measures (PROMs) and standardized clinical assessment instruments can improve the diagnostic accuracy and follow-up of the disease course.3 Some PROM questionnaires addressing LPR have been developed in the last 20 years. Among these, the most popular and relevant ones are the Reflux Symptom Index (RSI),13 which considers clinical symptoms, and the Reflux Finding Score (RFS),14 which assesses inflammatory signs at the laryngoscopy, both translated into and validated for Brazilian Portuguese.15–17 However, the main limitation of these instruments are the lack of specificity, as they seem to indicate laryngeal disease or inflammation, and not necessarily LPR.18,19
In this context, the Reflux Symptom Score-12 (RSS-12) was developed to aid the diagnosis and monitoring of LPR, including the impact on quality of life caused by the disease.20 The RSS-12 is a French, self-administered PROM consisting of 12 items and whose score varies from 0 to 300 (Fig. 1). It is the first clinical questionnaire based on the prevalence and relevance of the most common LPR symptoms. In addition, it is the only questionnaire that was created based on a cohort of patients diagnosed with LPR through MII-pH, including individuals with acid and non-acid reflux. It was created based on a cohort of 73 patients who completed the questionnaire at the time of LPR diagnosis using the MII-pH. It includes seven items on otorhinolaryngological symptoms, three items on digestive symptoms and two items related to respiratory symptoms. For each item, there is a frequency score that ranges from 0 to 5, where 0 means “I have not had this symptom in the last month” and 5 means, “I have had this symptom one or more times a day in the last month”, and a severity score that ranges from 0 to 5, where 0 corresponds to “the problem is not serious at all” and 5 to “the problem is very serious when it appears”. For each symptom, the frequency and severity scores are multiplied, yielding the symptom score (0‒25). The sum of the symptom score of the 12 items corresponds to the final score (0‒300). In addition, a quality of life impact score is included for each symptom (0‒5).
Original Reflux Symptom Score-12 instrument, written in French, extracted from the article by Lechien et al.20
The RSS-12 score > 11 is considered suggestive of LPR in a European population, with high sensitivity (94.5%) and specificity (86.2%).20 The higher the score, the greater the chance of LPR diagnosis and its impact on quality of life. The original French version also has excellent test-retest validity and reliability, with more discriminative properties compared to the RSI.20
With the consent and participation of the author of RSS-12, a study was proposed regarding the translation into Brazilian Portuguese and cross-cultural adaptation of the RSS-12, aiming to make the clinical diagnosis of LPR more specific and more cost-effective for the Brazilian population. The adaptation of previously validated scales and questionnaires is justified, since the adaptation is less expensive than the creation of a new instrument. Further, the use of equivalent instruments facilitates communication and the exchange of information within the scientific community.21
The aim of this study is to describe the process and methodology used for the translation into Brazilian Portuguese and cross-cultural adaptation of the French RSS-12 questionnaire.
MethodsThis is a cross-sectional study related the translation and cross-cultural adaptation of a health instrument. After the approval of the author of RSS-12, the process of translation and cross-cultural adaptation was carried out according to the methodology proposed by Guillemin, Bombardie and Beaton.22
For the cultural adaptation process, the Brazilian Portuguese versions of the questionnaire were applied to individuals aged 18 to 59 years, with symptoms and signs of LPR or GERD (dyspepsia, pharyngeal globus, heartburn, regurgitation, cough, throat clearing, atypical chest pain, hoarseness), who were referred by their physicians to undergo an esophageal function study using pHmetry, esophageal manometry or MII-pH at our institution, a private tertiary academic center. The sample was obtained by convenience. All consecutive patients who agreed to participate in the study were included, regardless of diagnostic confirmation of LPR or GERD through complementary exams. This study was submitted to the Research Ethics Committee of the institution and approved by it. The Free and Informed Consent form was applied to all participating individuals.
The translation and cultural adaptation of RSS-12 followed the steps described below:
1. Independent translation of the original RSS-12 in French into Brazilian Portuguese by two French translators, one a certified translator and another a gastroenterologist physician who was bilingual in Portuguese and French, without prior knowledge of the instrument.
2. Verification of the first provisional versions in Brazilian Portuguese by a panel of experts, consisting of a head and neck surgeon, two otolaryngologists and two gastroenterologists. The cultural adaptation of words, semantics and expressions was carried out to develop version 1 of RSS-12 in Brazilian Portuguese.
3. Pilot test 1: Application of version 1 of RSS-12 in Brazilian Portuguese to 30 patients, to check the understanding of the text and table format. The questionnaires were self-filled. The volunteers were instructed to inform the interviewer, after completing the questionnaire, if they did not know a certain word or did not understand its meaning and classify the questions and scores as easy or difficult to answer. Difficulties in understanding the items or specific terms by the participants were noted and the necessary modifications were made by the panel of experts.
Adaptations were made and version 2 of the RSS-12 in Brazilian Portuguese was prepared by the panel of experts5. Pilot test 2: Application of version 2 of the RSS-12 in Brazilian Portuguese to 23 patients, to verify the understanding of the text and table format. The questionnaires were self-filled. Difficulties in understanding the items or specific terms by the participants were noted and the necessary modifications were made by the panel of experts.
6. Back-translation of version 2 of RSS-12 in Brazilian Portuguese into French by two translators, one French individual, bilingual in Portuguese and French, and one Portuguese/French translator, without prior knowledge of the instrument.
7. Verification of the back-translation of version 2 of the RSS-12 in Brazilian Portuguese into French by the author of RSS-12 (Jerome R. Lechien). If the assessment was satisfactory, version 2 of the RSS-12 in Brazilian Portuguese would be considered viable for the continuation of the study; in the event of low agreement, the initial stages would be repeated.
8. Consensus on the modifications proposed by the author of the original version and Brazilian experts to create the final version of the RSS-12 in Brazilian Portuguese (RSS-12 PT-BR).
9. Application of the RSS-12 PT-BR questionnaire to 25 patients to check the quality of the translation and the adequacy of the interpretation of the questions, objectivity, clarity and consistency of answers.
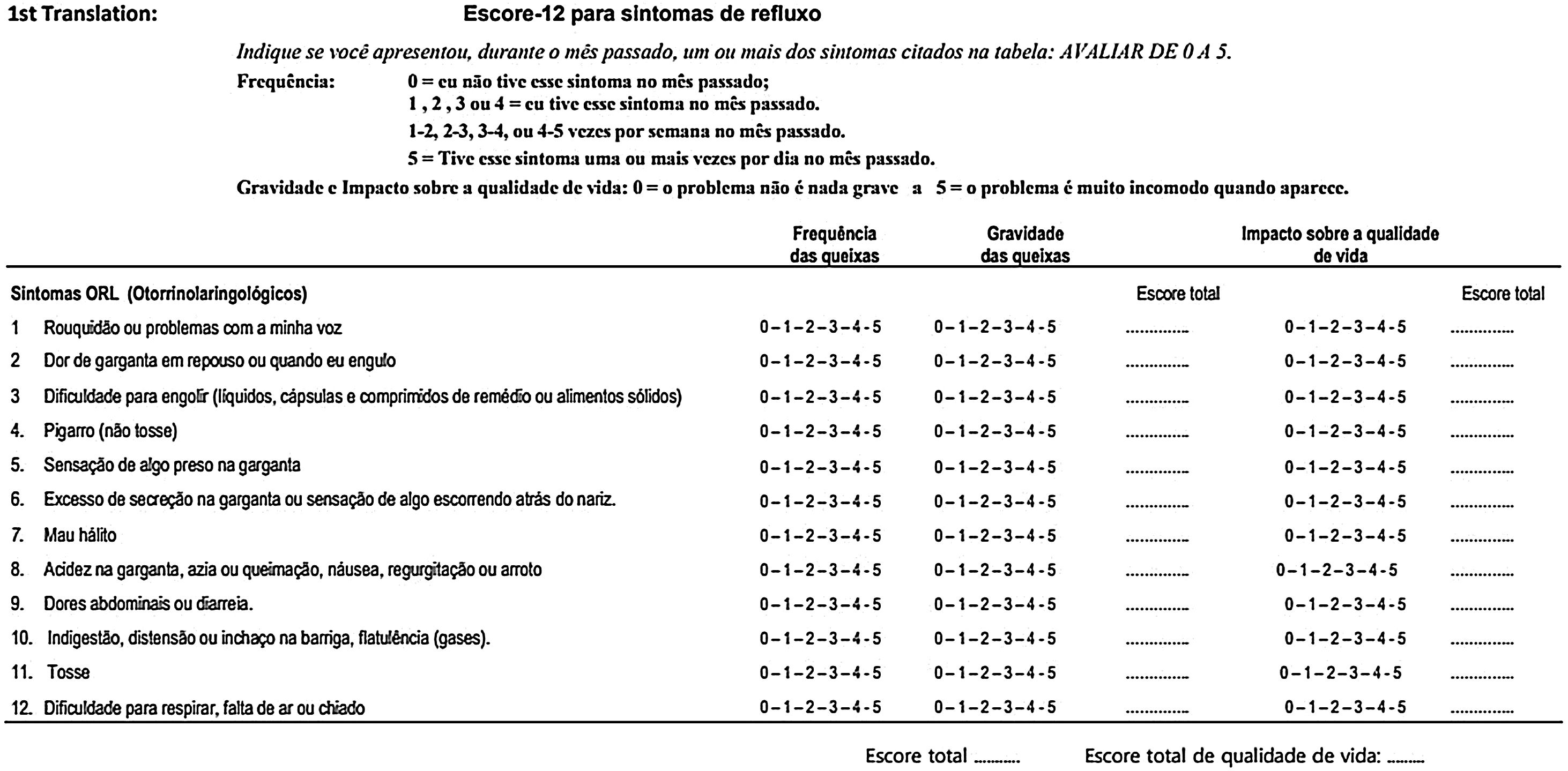
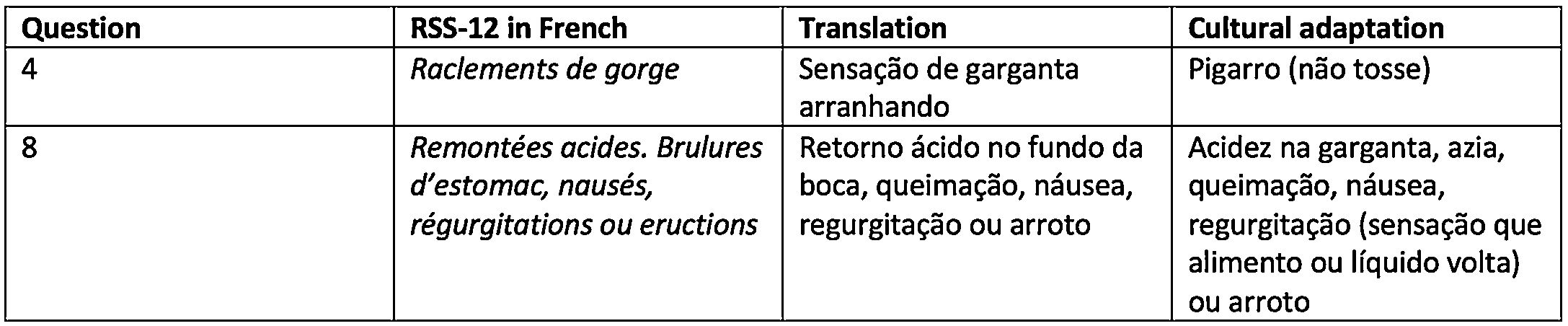
ResultsVersion 1 of the RSS-12 in Brazilian Portuguese (Fig. 2) had several adaptations proposed by the panel of experts, who, working in different specialties, had different perceptions regarding the creation of the items and choice of words and expressions of the questionnaire. Fig. 3 exemplifies items that required cultural adaptation into Brazilian Portuguese.
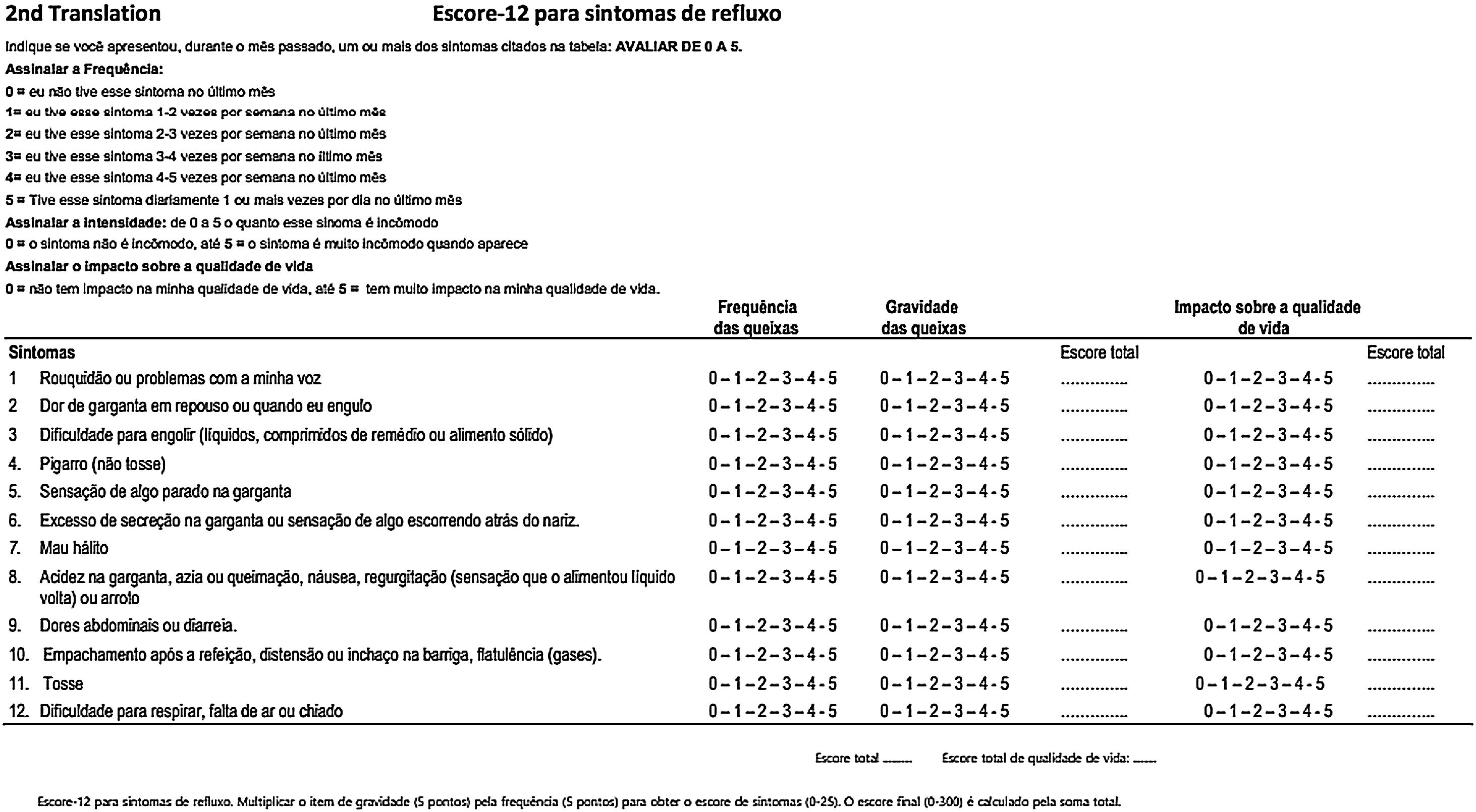
In the pilot test 1, version 1 of the RSS-12 in Brazilian Portuguese was self-applied and completed by 30 patients. There were no difficulties in understanding regarding any of the 12 symptom items. None of the participants classified the content as irrelevant or offensive. However, there was some difficulty interpreting the recommendation to indicate the symptom frequency score (0‒5), a legend that appears at the beginning of the questionnaire. Fifteen patients (50%) claimed to have difficulty, leaving the completion of the frequency score incomplete. Therefore, we chose to modify the frequency score presentation form, separating more clearly the frequency and the score to be recorded by the patient, without, however, modifying the substance of the original questionnaire (Fig. 4).
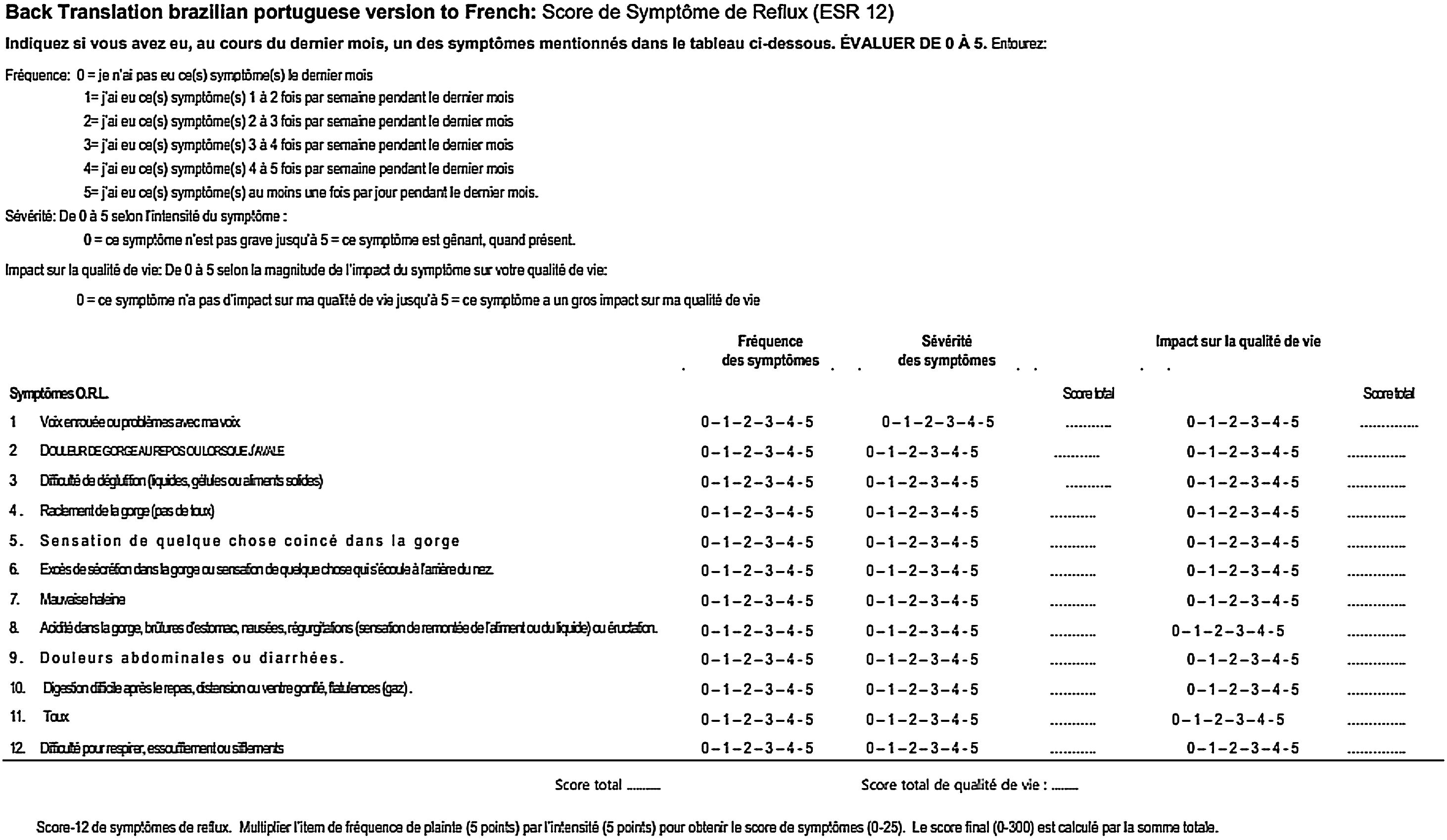
Version 2 of the RSS-12 was self-filled by another 23 patients, who answered the questionnaire completely and without any difficulties. There were no requests to repeat any of the questions. This version was back-translated from Brazilian Portuguese into French and sent to the main author and creator of the RSS-12, highlighting the modifications made in relation to the presentation of the frequency score that we proposed (Fig. 5).
The author of RSS-12, Jerome R. Lechien, had access to the two versions in Brazilian Portuguese and the back-translation from Brazilian Portuguese into French and considered both the form and the translation from Portuguese into French correct and ready to be applied to a broader population for further validation. Thus, it was not necessary to revise any item of version 2 of RSS-12 PT-BR and so, it was approved as the final version of RSS-12 by the panel of experts.
The sample to which RSS-12 PT-BR was administered consisted of 23 patients, 13 (56.5%) female and 10 (43.5%) male. The age of the sample ranged from 18 to 69 years, with a mean of 46.2 ± 12.7 years and a median of 42 years.
DiscussionThe diagnosis of LPR is considered difficult, since the symptoms and signs are nonspecific and there is no gold standard complementary test. The RFI and RFS questionnaires, although widespread, are considered not very specific and were constructed based on the diagnosis of LPR by pHmetry, a method that does not take into account non-acidic and gaseous reflux, responsible for about half of LPR events. In this context, the RSS-12 was chosen to be translated and adapted into Brazilian Portuguese, as it is a self-administered, quick and easy to understand questionnaire, with high sensitivity and specificity for the diagnosis of LPR. The method proposed by Guillemin, Bombardie and Beaton was used,22 which has been acknowledged and used by several authors,15,23,24 and consists of five stages: translation, synthesis, back-translation, review committee and application.
It is important to differentiate the terms “translation” and “cross-cultural adaptation”. The translation corresponds to the translation of the instrument from the original language into the target language. The cross-cultural adaptation is the development of versions of an instrument that are equivalent to the original, but linguistically and culturally adapted to a context that is different from the original one.21 Attaining cultural equivalence is an important and essential step for an instrument that was originally developed in another language to be used in a different language and culture, so that there are no barriers between the instrument and its addressed population.
The panel of experts chose to make some adaptations to the literal translation. As for the temporal orientation of symptom occurrence, “last month” was used instead of “the past month”, as it better expresses events that happened in a more immediate period in relation to the present time. As for severity, the term “problem” was replaced by “symptom”, which refers to the indication of the table itself containing the 12 items to be evaluated. The cultural adaptation of symptoms had a significant intervention in symptom “4”, whose literal translation resulted in “scratchy throat sensation”. In the original work by Lechien et al.,20 in which the group of authors translated it into English, the expression “clearing throat” appears, which, translated into Portuguese, results in “pigarro”. For this reason, we chose to use this word, frequently reported by patients under investigation for LPR.
Specific recommendations were also added for completing the quality of life assessment, which is not present in the original version. We believe that it is important to present the specific quality of life scale, and not just a parallel with the indication of severity, in which “0” means absence of severity and impact on quality of life and “5” means very severe symptom, with a great impact on the worsening of the quality of life.
The patients completed the questionnaire immediately before their examinations. The physicians in charge of supervising the self-application instrument performed the immediate assessment of the understanding of symptoms and comprehension difficulties and were not asked about the meaning of words or symptoms presented in the RSS-12, which represents an adequate translation and cultural adaptation of the evaluated items. The panel of experts met at two different times to evaluate the translation versions, but in relation to the translation of the 12 symptom items, there were no changes between the first and second versions.
The back-translation of version 2 of RSS-12 in Brazilian Portuguese into French was sent by e-mail to the main author of the questionnaire, Jerome R. Lechien, pointing out the change in the presentation of the scores. Lechien considered the created version to be adequate and did not propose any changes. For this reason, version 2 of RSS-12 in Brazilian Portuguese was considered as the final version by the panel of experts, abbreviating the proposed translation steps.
We understand that questionnaires that are self-filled by patients can represent a barrier to patients with visual difficulties or those who are illiterate. These difficulties must be carefully considered by the health team when such limitations are identified. We understand that the active application of this questionnaire by the physician or another member of the multidisciplinary team does not lead to interference in the measured results. However, in the assessed population, no difficulties were observed regarding linguistic or form comprehension, or visual disturbances that prevented the reading. We emphasize that, once this questionnaire has been validated for the Brazilian population, the assisted application may represent a solution for this particular population.
This questionnaire has already been translated and validated into Korean.25 It is currently in the final phase of validation into English and we have started the validation process for the Brazilian population.
The present article is the result of the collaboration of translators, physicians and patients. We understand that the development of this project will shorten the task of other groups or professionals to validate this questionnaire into Brazilian Portuguese, collaborating to the diagnosis and follow-up of patients with LPR, in addition to being an alternative for future studies in this very challenging context of patients with LPR.
ConclusionThe Brazilian Portuguese version of the instrument, called Reflux Symptom Score-12 PT-BR, has shown good comprehension and linguistic, conceptual and content equivalence, in relation to the original Reflux Symptom Score-12.
Conflicts of interestThe authors declare no conflicts of interest.
The authors have no funding or financial relationships to disclose.