Cluster headache is considered a trigeminal autonomic cephalalgia and may present with characteristic symptoms of sympathetic/parasympathetic activation on the affected side of the face, such as nasal discharge, tearing, and conjunctival injection. Invasive therapies targeting the sphenopalatine ganglion have been performed in these headache syndromes and can have a medication-sparing effect, especially in refractory, difficult-to-manage cases. The gate control theory of pain suggests that electric pulses delivered to nerve tissues can modulate neuronal activity, thus aiding in management of nociceptive or neuropathic pain, and studies have demonstrated the efficacy and safety of sphenopalatine ganglion neurostimulation. Within this context, we sought to assess the feasibility of a new surgical technique for neurostimulation of the sphenopalatine ganglion in a cadaver dissection model.
MethodsThe technique was developed through dissection of two cadaver heads. We divided the procedure into two stages: an endonasal endoscopic approach to expose the sphenopalatine ganglion and confirm electrode placement, and a cervicofacial approach to introduce the electrode array and position the internal pulse-generator unit. Computed tomography was performed to confirm implant placement at the end of the procedure.
ResultsThe pulse-generator unit was successfully placed through a retroauricular incision, as is already standard for cochlear implant placement. This should reduce the incidence of perioperative sequelae, especially pain and swelling in the oral region, which are a common complication of previous approaches used for this purpose. Control imaging confirmed proper electrode placement. The device used in this study allows the patient to modulate the intensity of the stimulus, reducing or even obviating the need for drug therapy.
ConclusionThe novel technique described herein, based on percutaneous access guided by transmaxillary endoscopy, can provide great precision in electrode array positioning and decreased perioperative morbidity, combining the advantages of endoscopic approaches with those of the retroauricular route.
Level of evidence3.
Primary headaches are characterized by their poorly defined causes and by not being a consequence of any underlying disease. Their chronic, severe nature can impair the quality of life of sufferers, with episodes often requiring medical treatment with abortive drugs (such as injectable triptans or supplemental oxygen) and, in the most extreme cases, prophylactic treatment with psychotropic drugs, such as mood stabilizers and anticonvulsants. Other classes may also be useful in the treatment of primary headache, such as corticosteroids, calcium channel blockers, and melatonin; however, these have potential cardiovascular and metabolic adverse effects, such as weight gain, hypo- and hypertension, AV block, and myocardial infarction.1,2
One of the subtypes of primary headache is cluster headache, characterized by unilateral periorbital pain lasting 15 min–3 h, following a circadian or circannual pattern, and affecting mainly males. Cluster headache is considered a Trigeminal Autonomic Cephalalgia (TAC) and may present with characteristic symptoms of sympathetic/parasympathetic activation on the affected side of the face, such as nasal discharge, tearing, and conjunctival hyperemia.1
Considering the challenges and limitations of pharmacological treatment, invasive therapies have been described with the aim of reducing the use of medications and the impact of their adverse effects, especially in refractory, difficult-to-manage cases. These combine the benefits of continuous, modulable therapy with those of a single surgical procedure, thus ensuring long-term treatment adherence, as well as a favorable cost-effectiveness ratio, since the prolonged use of medications is common in these conditions. In this sense, some procedures have targeted the Sphenopalatine Ganglion (SPG) to treat headache, including percutaneous alcohol neurolysis, local application of lidocaine or corticosteroids, and radiofrequency ablation. Neurostimulation of the SPG, although only recently described, has become an interesting alternative.1–4
ObjectiveTo describe the feasibility of a new surgical technique for neurostimulation of the sphenopalatine ganglion.
MethodsThe technique was developed by means of cadaver dissection at laboratory. Two frozen cadaver heads, sectioned at neck height, were used. The major arteries and veins of both specimens were cannulated with Foley catheters, irrigated with water, and subsequently plastinated with red and blue latex, respectively.
Karl Storz-brand surgical instruments (Culver City, CA, USA) were used, including 0°, 30°, and 45° endonasal endoscopes, skull-base dissection forceps, and an Aida® endoscopy/image documentation system. Motorized instrumentation was used, with burs and shaver blades coupled to a UniDrive® control unit.
Computed tomography of the face with slice thickness 0.5–1 mm was performed to detect any anatomical variations in the skeletal parameters of each specimen, as well as to confirm implant placement at the end of the procedure.
Description of operative technique. We divide this procedure into two routes of approach: the transnasal endoscopic route and the cervicofacial route.
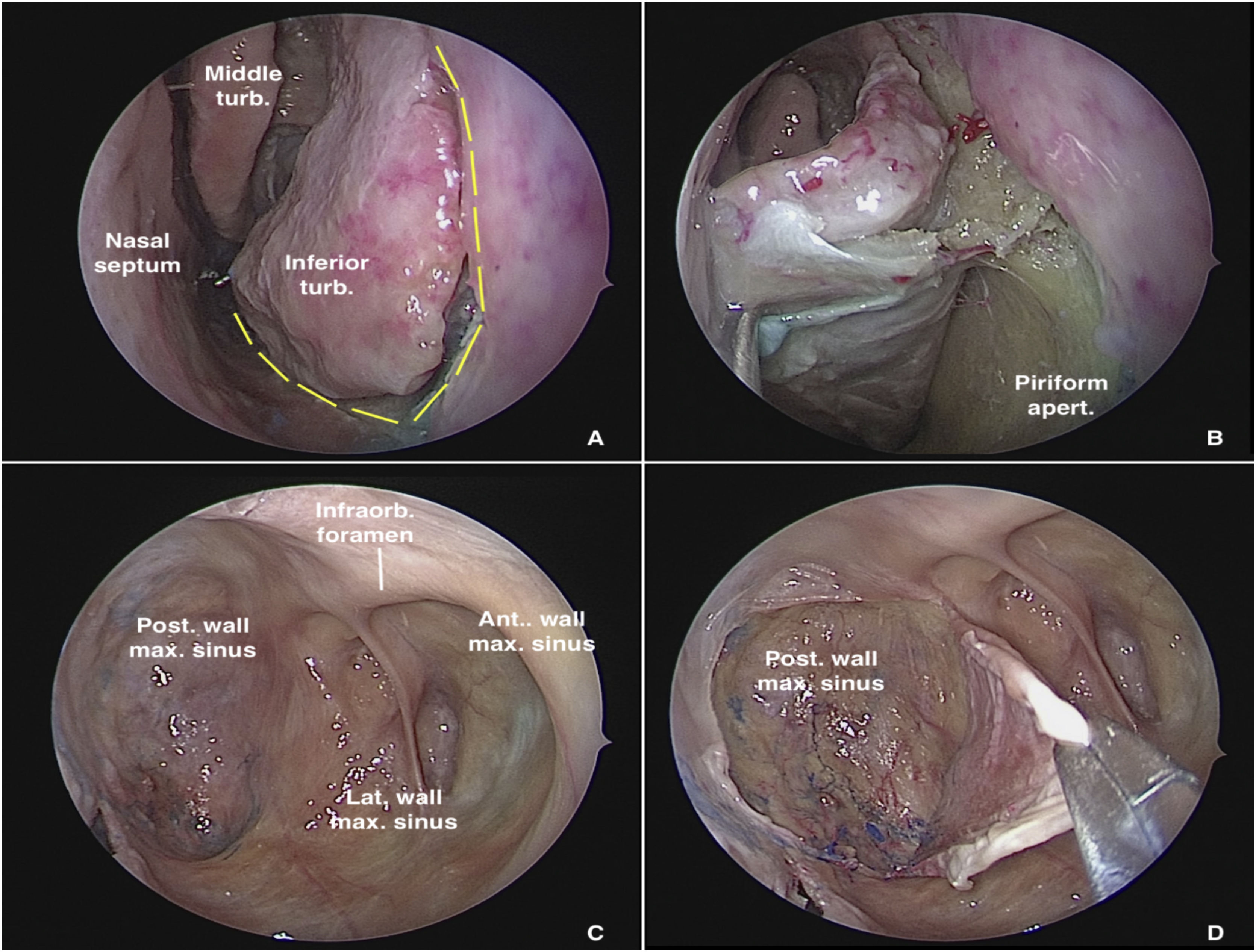
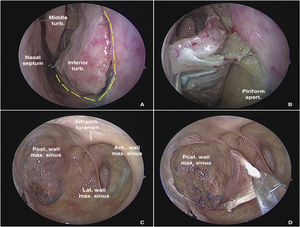
Dissection begins via the transnasal endoscopic route, using the reversible endoscopic medial maxillectomy technique as described by Tepedino et al.5 The procedure is performed through the nasal cavity ipsilateral to the most symptomatic side. The uncinate process is resected, followed by wide maxillary antrostomy after identification of the natural ostium of the maxillary sinus, providing good visualization of the posterior wall of the sinus. After adequate identification of the maxillary line, a fine-tipped monopolar cautery is used to make an oblique pre-lacrimal incision, starting 0.5 cm anterior to the superior portion of the nasolacrimal duct and continuing inferiorly, anterior to the inferior turbinate, up to the floor of the nose, through the piriform aperture (Fig. 1A). The incision is then extended posteriorly through the floor of the nose to the posterior portion of the inferior turbinate. Subperiosteal undermining is performed to expose the frontal process of the maxilla (Fig. 1B). A straight osteotome is then used to fracture the maxilla along the previously demarcated line. The entire medial wall of the maxilla, including the nasolacrimal duct and inferior turbinate, is displaced medially, providing ample exposure of all maxillary sinus walls, even with a 0° endoscope (Fig. 1C).
Transnasal approach to the left maxillary sinus with a 0° endoscope. (A) Incisions on the lateral wall of the nose for medial displacement of the medial wall of the maxillary sinus. (B) Exposure of the piriform aperture and mucosal undermining before osteotomy. (C) Endoscopic control of all maxillary sinus walls. (D) Detachment of the mucosa from the posterior wall of the maxillary sinus for subsequent bone removal and access to the pterygopalatine fossa.
If a well-pneumatized lacrimal recess is found, as is estimated to occur in approximately 70% of cases, a reversible medial maxillectomy can be performed, and at the end of the procedure, the medial wall of the maxilla is replaced, and the mucosal incisions are closed with two Vicryl 3‒0 sutures. When the nasolacrimal duct is too close to the anterior wall of the maxillary sinus, there is a high risk of injury to the duct with the technique described above. In this situation, we suggest performing a post-lacrimal maxillectomy, preserving the inferior turbinate.6
Once the posterior wall of the maxillary sinus has been fully exposed, a new mucosal incision in the shape of a “square C” is made with a fine-tipped diathermy pencil, creating a mucosal flap which is pedicled in its most lateral region and undermined from medial to lateral, allowing exposure of the bony wall (Fig. 1D). Osteotomies are performed over the line of the incision and the bone is removed, exposing the periosteum of the posterior wall of the maxillary sinus. The periosteum is carefully incised with a sickle knife, exposing the fat and other contents of the Pterygopalatine Fossa (PPF).
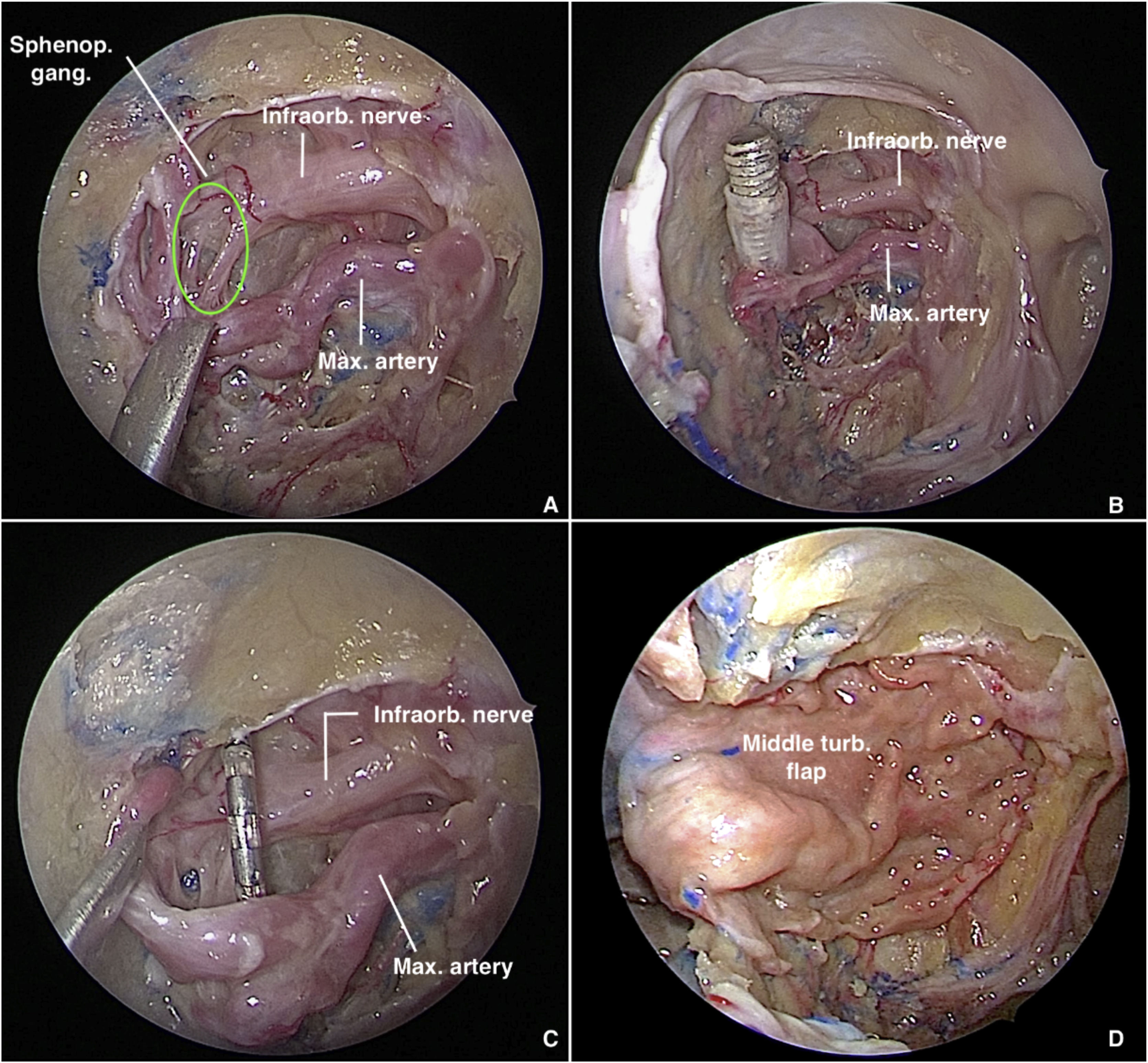
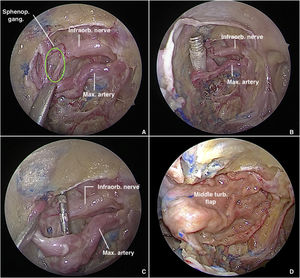
PPF fat is retracted and partially removed with bipolar cautery and blunt dissection to expose the vascular branches, which must be carefully dissected and preserved, under light traction, to expose the neural structures of the SPG (Fig. 2A). To delimit the SPG precisely and achieve accurate electrode placement, the following landmarks must be identified: the foramen rotundum (lateral), the pterygopalatine foramen (medial), and the maxillary artery and its branches (anterior).7 Depending on the severity of bleeding or the amount of fat in the PPF, exact delimitation of the ganglion can be challenging. A valuable tip to locate it is to follow the divisions of the vidian and maxillary nerves after they emerge through the pterygoid canal and foramen rotundum, respectively. Then, dissection of the middle turbinate is performed and a pedicled flap is raised from the lateral wall of the nose. The flap is placed in the rhinopharynx, taking care not to twist the arterial pedicle.
Transmaxillary approach to the pterygopalatine fossa with a 0° endoscope. (A) Sphenopalatine ganglion in green. (B) Trocar tip positioned in the pterygopalatine fossa after introduction via the external route. (C) Electrode carefully placed anterior to the sphenopalatine ganglion. (D) Pterygopalatine fossa covered with a middle turbinate flap.
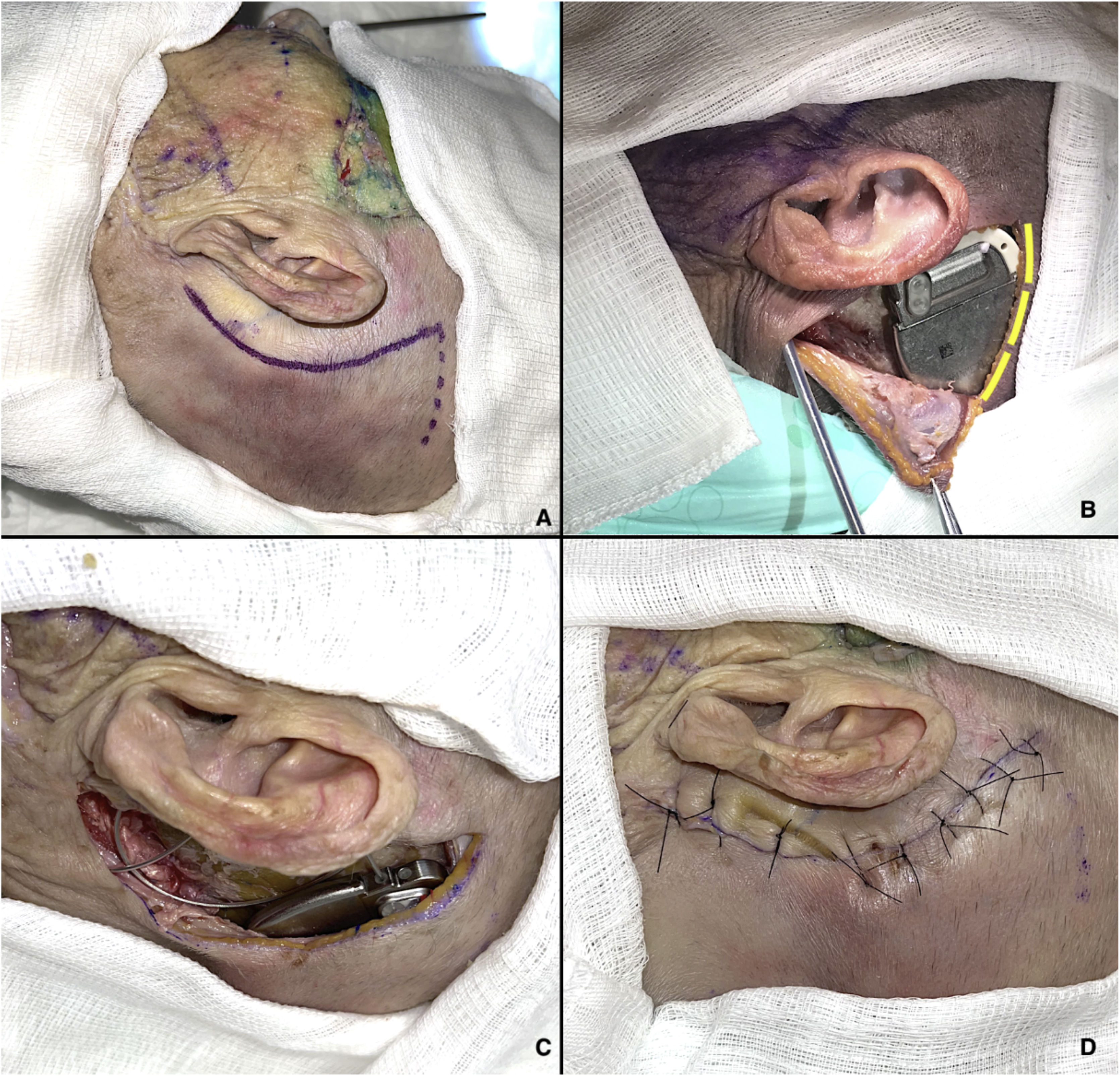
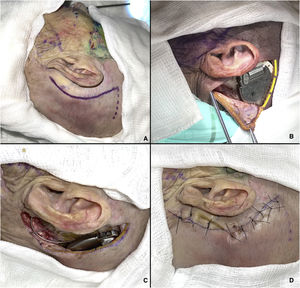
The cervicofacial route begins with an approximately 7-cm arcuate retroauricular incision to fashion a subperiosteal pocket, resembling the standard approach for cochlear implant placement, which will house the internal pulse generator.8 This incision is extended inferiorly towards the tip of the mastoid, anterior to the sternocleidomastoid muscle, through which a trocar is passed to direct the electrode array to the PPF (Fig. 3A and B). If necessary, a pocket can be drilled into the outer cortical table of the skull for better fit of the pulse generator. At the end of the procedure, a layered closure is performed, suturing the muscles with 3–0 Vicryl and the skin with 4–0 nylon.
Cervicofacial route. (A) Markings for retroauricular incision. (B) Internal pulse generator in place; an additional incision has been made (yellow lines) for better visualization of the device. (C) Electrode array coupled to internal pulse generator. (D) Closure of retroauricular incision.
The trocar catheter enters the PPF through its lateral communication with the Infratemporal Fossa (ITF), tangential to the lateral plate of pterygoid process, after passing through the masticator space and traversing fibers of the lateral pterygoid muscle. Once within the PPF (Fig. 2B), under endoscopic visualization, the trocar is removed, the catheter remains in place, and the electrode array is introduced through it. Finally, the array is positioned anterior to the SPG (Fig. 2C), the ganglion itself being carefully avoided, and Surgicel® is used to aid in fixation. Finally, the ipsilateral middle turbinate flap is rotated medial to lateral through the maxillectomy to cover the electrode array and the surgical defect in the posterior sinus wall (Fig. 2D). The previously reflected mucosa of the posterior wall of the maxillary sinus is used to cover the exposed bone after flap rotation.
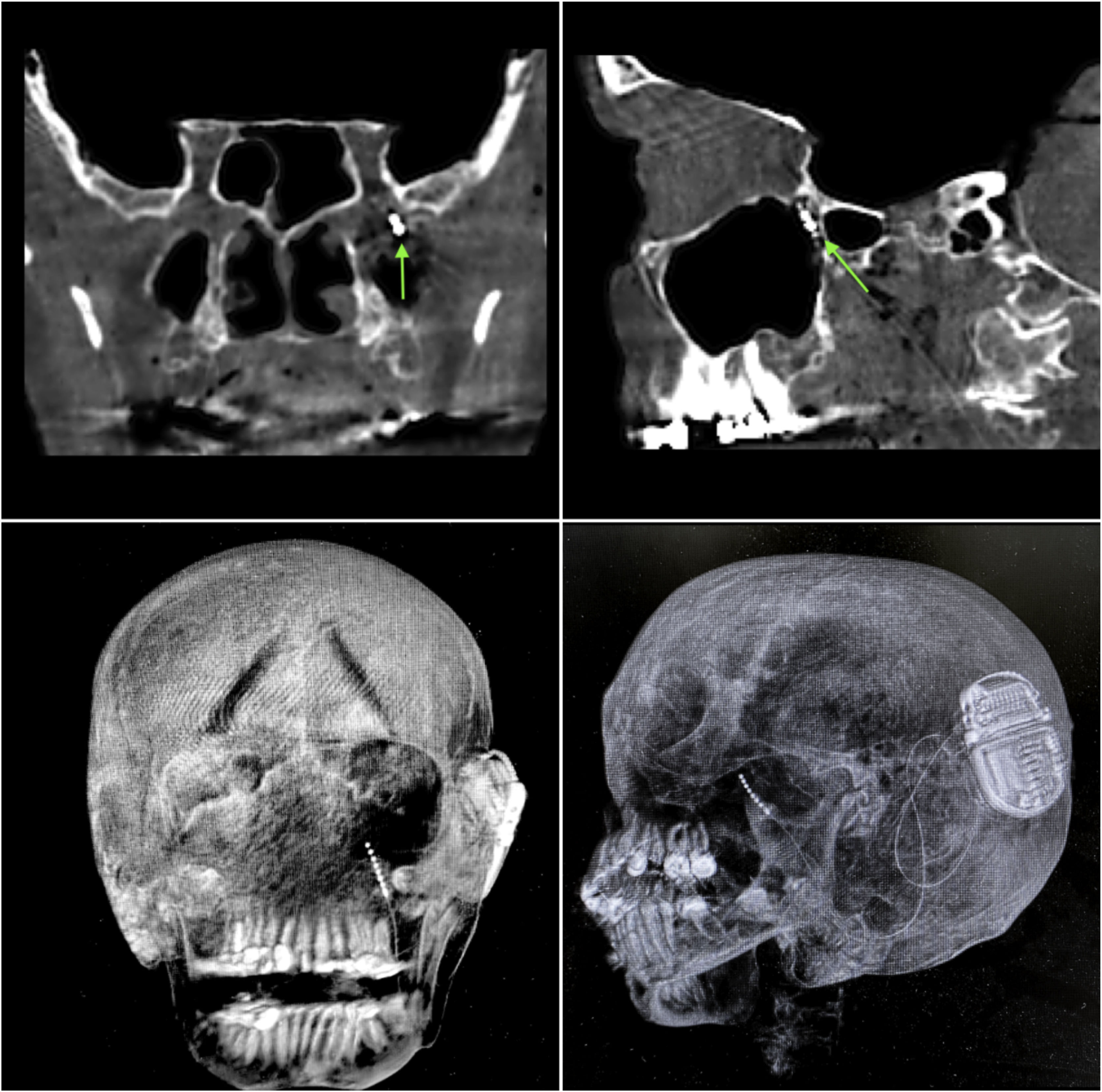
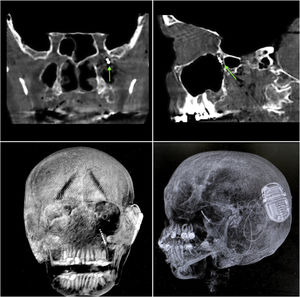
ResultsIn both cadavers, it was possible to identify the sphenopalatine ganglion after endonasal endoscopic dissection of the pterygopalatine fossa, with preservation of vascular structures (Fig. 2). Electrode placement was confirmed by direct endoscopic visualization as well as computed tomography with coronal, sagittal, axial, and three-dimensional volume-rendering reconstructions (Fig. 4A–D). The internal pulse generator was perfectly accommodated in the retro-auricular region.
Control computed tomography obtained after neurostimulator implantation. (A and B) Bone window, coronal and sagittal sections respectively; green arrow represents the location of the electrode tip within the sphenopalatine ganglion. (C and D) Three-dimensional reconstruction, allowing visualization of the entire electrode path and location of the internal pulse generator.
Because of its repetitive nature and extreme severity, cluster headache is also known as the “suicide headache”. Although rare, with an annual prevalence of approximately 0.12%, this condition has an enormous impact on quality of life. Two distinct clinical forms are recognized: chronic and episodic. The episodic form is characterized by symptoms with a variable duration of 7 days to 1 year, with asymptomatic periods lasting at least 1 month between flares. The chronic form, accounting for 15% of cases, is characterized by pain-free periods lasting less than 1 month throughout the course of a year, or by no pain-free periods at all.1
For over 100 years, the SPG has been believed to play a role in the genesis of pain and autonomic symptoms in cluster headache. Activation of the trigeminal-autonomic reflex would cause pain through potent dilatation of meningeal and cerebral vessels, also triggering reflex connections in pons neurons and thus increasing SPG-mediated parasympathetic activity. This positive feedback system can initiate and/or sustain pain.1–3
The SPG is a complex parasympathetic autonomic neural structure of the face, located in the pterygopalatine fossa. It is responsible for glandular innervation and vascular tone in the nasal cavities, paranasal sinuses, lacrimal glands, and palatine glands, in addition to providing sensory innervation to the nasal mucosa. The PPF is delimited by bony structures: the posterior wall of the maxillary sinus (anteriorly); pterygoid process and greater wing of the sphenoid (posteriorly); body and greater wing of the sphenoid and inferior orbital fissure (superiorly); perpendicular plate of the palatine bone (medially); and pterygomaxillary fissure (laterally), which communicates the PPF and ITF. Inferiorly, it is bounded by the palatine canal, with the greater and lesser palatine foramina. The contents of the PPF consist largely of fatty tissue, as well as terminal branches of the maxillary artery (located anteriorly) and autonomic nerves (posteriorly). It receives fibers of the vidian nerve via the pterygoid foramen, medially, while the maxillary (V2) division of the trigeminal nerve reaches it through the foramen rotundum, more laterally, and continues as the infraorbital nerve after issuing sensory branches that course through the SPG.3
Due emphasis should be given to the vascular anatomy of the PPF, since the innovation of the procedure describes herein lies precisely in its safety for the prevention and endonasal endoscopic control of arterial injuries that might result from the retromandibular puncture performed to allow passage of the electrode. Vascular structures are normally located in an anterior position in relation to neural structures within the PPF, so that careful endoscopic dissection and debulking of fat tissue in the PPF (i.e., skeletonization of any arterial branches) must be performed before puncture, allowing optimal visualization and handling of the electrode tip by the surgeon in order to position it precisely over the SPG.
The maxillary artery (terminal branch of the external carotid artery) enters the PPF through the pterygomaxillary fissure, continuing its course which began in the ITF. Distally, the maxillary artery divides further, giving rise to the descending palatine artery and the sphenopalatine artery, which in turn subdivides into the posterior lateral nasal arteries and the posterior septal artery.9
Anatomical variants both in the neural and in the vascular structures are extremely common in this narrow fossa; therefore, endoscopy-assisted approaches are safer, due to the magnification and wide-angle effect provided by the optics. This ensures the preservation of neurovascular structures during the procedure and allows their direct manipulation (cauterization or clipping) for immediate hemostatic control in case of iatrogenic injury.
Recent research with implantable microstimulators showed that 68% of patients experienced clinically relevant improvement in their symptoms during the study, achieving relief during at least 50% of episodes treated with full stimulation, as well as a reduction of at least 50% in episode frequency compared to baseline.1 Ancillary analyses demonstrated that more patients had pain relief and/or resolution within 15 min and sustained pain relief for 1 h in at least 50% of episodes comparing stimulator versus sham treatment.2
THE Intellis-97716 system® (Medtronic, Dublin, Ireland) was designed to treat lower-back and sciatic pain by spinal neurostimulation. It delivers electrical pulses to the spinal cord to modulate neuronal activity. This reduces pain sensation, in accordance with the “gate control theory” of nociceptive or neuropathic pain.10,11 The system consists of the following items: implant (internal pulse generator, cable, electrode arrays); external programmer/controller unit, through which the patient can check battery levels as well as adjust stimulation parameters and select pre-defined therapy programs; telemetry unit; wired antenna; and a transcutaneous induction charging system. The battery of the pulse generator recharges transcutaneously through a magnet, using an antenna wired to the patient’s programmer/controller unit.10
At the end of its service life, which depends on how heavily the patient uses the system and on the number of charging cycles, a new percutaneous approach would be required, but only to access the Behind-The-Ear (BTE) unit and replace the pulse generator; no manipulation of the cables, electrode array, or PPF would be necessary. One advantage of the Intellis implant is its MRI compatibility (SureScan technology®), so that the patient would not need explant surgery to undergo neuroimaging (which is very commonly necessary in patients with chronic, severe headaches).10
Another advantage is the large number of predefined programs that can be saved,12 customizing parameters such as stimulus intensity, pulse rate, amplitude, and number of repetitions, in a patient-controlled manner. This feature is even more important in patients with cluster headaches, whose attacks can vary in severity and duration.
The spine implant features so-called AdaptiveStim® mode, which detects the position of the patient’s body and allows the programming to be adjusted accordingly. This could be an interesting feature in the SPG implant as well, as changes in head position frequently occur throughout the day and night. Patients themselves can modulate stimulus intensity, on demand — a personalized approach which can reduce drug use or even make it altogether unnecessary.10
Some research, such as that conducted by Assaf et al. and Goabsy et al., has already demonstrated the efficacy and safety of SPG neurostimulation.1,2 In these studies, however, access was achieved via the transoral subgingival approach, bordering the maxilla. Perioperative sequelae were as expected for an oral-maxillofacial procedure, such as sensory disturbances, pain, and local swelling. In the approach described herein, the only external incision performed is on periauricular skin, with no manipulation of oral tissue. We hope that this will decrease the incidence of perioperative sequelae, especially regarding pain and edema, which are only infrequently observed in other procedures performed via the same access route, such as cochlear implantation and reversible endoscopic medial maxillectomy.5,12
A CT scan of the face is obtained with a slice thickness of 0.5–1 mm to detect anatomical variations in bony structures, as well as to evaluate pneumatization of the lacrimal recess of the maxillary sinus, which determines whether transmaxillary access will be performed by reversible endoscopic medial maxillectomy or endoscopic post-lacrimal medial maxillectomy. Beyond detailed anatomical evaluation, CT can rule out preexisting bone defects and primary skeletal diseases, as well as diseases of the paranasal sinuses, which could contraindicate the procedure due to the risk of implant displacement and/or biofilm formation, respectively.
Some studies suggest that puncture be performed under fluoroscopic guidance in order to guide the tip of the implant through skeletal landmarks, thus preventing creation of a false path and reducing the risk of iatrogenic injury.1 In our procedure, we considered this step unnecessary, since electrode positioning occurs under direct endoscopic visualization, with no risk of incorrect positioning. In addition, a blunt-tipped trocar is used during the cervicofacial approach, and its course takes it through the masticator space, where only muscle fibers are traversed — lateral to the parapharyngeal and vascular spaces, which contain the major cervical vessels.
Risks inherent to the procedure can be classified as implant-related or approach-related. Implant-related risks include biofilm formation, insufficient stimulation, pain at the pulse generator site, malfunction, cable or generator rupture or disconnection, fibrosis, or electrode array migration. Approach-related risks include peripheral facial palsy, puncture site hematoma, surgical site infection, vascular injury (especially of branches of the external carotid), epistaxis, cervical hematoma, orbital hematoma, and globe perforation.
Postoperative limitations would involve contraindications to use of the monopolar cautery in subsequent procedures — a limitation inherent to most electrical implantable devices. Restrictions to strong electromagnetic fields, such as metal detectors at airports and some MRI scanners, are also worth noting.
ConclusionNeurostimulation of the SPG for the treatment of primary headaches has already proven effective and feasible in some studies. The present article introduces a new approach, based on percutaneous access guided by transmaxillary endoscopy, which is associated with great precision in electrode array positioning and decreased perioperative morbidity, combining the advantages of endoscopic approaches and the retroauricular route.
Conflicts of interestThe authors declare no conflicts of interest.
Peer Review under the responsibility of Associação Brasileira de Otorrinolaringologia e Cirurgia Cérvico-Facial.