Weight loss is one of the most often prescribed treatments to reduce the level of sleep apnea severity; however, objective assessment of airway alterations after loss of weight has only been studied in the last decades. This study aimed at evaluating alterations after weight loss reported in the literature.
MethodsA literature review was performed in the medical databases: PubMed, Web of Science, Scopus and Embase. A total of 681 articles were found in the databases and after evaluation only 10 studies were selected for data extraction.
ResultsMost studies observed an increase of the area in the retropalatal region; some indicating that this increase occurred mostly in the lateral pharyngeal region. Studies with volumetric reconstruction showed a significant reduction in parapharyngeal fat deposits, lateral wall and tongue fat, and volumetric reduction in all soft tissues of the pharynx, pterygoid and genioglossus muscles. Studies evaluating craniofacial bone structures showed a reduction in the airway height by bringing the hyoid closer to the posterior nasal spine and a reduction in the distance from the hyoid to the chin.
ConclusionThere is a limited number of studies with a good level of scientific evidence evaluating changes in the upper airways after weight loss and how these changes impact obstructive sleep apnea. The studies included in this review indicate that weight loss increases the airways space by reducing the volume of the parapharyngeal structures, particularly at the retropalatal site, where there is an apparent gain in the lateral area of the airway and hyoid relocation.
Obstructive Sleep Apnea (OSA) is characterized by upper airway obstruction during sleep, resulting in periods of apnea, oxyhemoglobin desaturation, and frequent awakenings, with consequent daytime sleepiness.1 Among the factors associated with sleep apnea, obesity is identified as one of the main causal and perpetuating factors.2 In an epidemiological study carried out in the city of São Paulo, in which the prevalence of OSA was 32.8%, 60% of the volunteers were overweight.3
The mechanisms involving obesity and OSA are not yet fully elucidated. The reduction in upper airway volume due to the increase in adipose tissue in the cervicofacial region has been demonstrated in imaging studies.4,5 Another mechanism would be the increase in visceral fat, causing a reduction in lung volume, which would lead to a reduction in the upper airway volume, reducing caudal tracheal traction and supporting increased pharyngeal collapsibility.6–8
Since obesity is an important risk factor for OSA,9 loss of weight is a recommended treatment, mainly through lifestyle interventions, such as a calorie-restricted diets, physical exercises and education about OSA and obesity.10 Studies have already shown that the reduction of the Body Mass Index (BMI) can reduce OSA severity, with a decrease in the Apnea-Hypopnea Index (AHI) in addition to the improvement in metabolic, cardiovascular and quality of life outcomes.9–14 Evidence has shown that, along with weight loss, there is a reduction in airway collapsibility due to metabolic alterations15 and anatomical changes resulting from weight loss.16,17
To better elucidate which anatomical changes in the upper airway occur with body mass reduction, this study aimed to review the literature on the subject.
MethodsUsing the guiding question “What are the changes in the upper airway after weight loss?” the research was structured following the acronym PICO as follows: P ‒ Obese; I ‒ Weight loss, C ‒ Comparison with baseline and O ‒ Anatomical changes in the upper airway.
Literature review was performed in the medical databases: PubMed, Web of Science, Scopus and Embase, having as descriptors to be evaluated in (titles, subject and abstract), according to those reported in health sciences in English: “upper airway” AND “Sleep Apnea, Obstructive” OR “Apnea, Obstructive Sleep” OR “Apneas, Obstructive Sleep” OR “Obstructive Sleep Apnea” OR “Obstructive Sleep Apnea Syndrome” OR “Obstructive Sleep Apneas” OR “Sleep Apnea Hypopnea Syndrome” OR “Sleep Apnea Syndrome, Obstructive” OR “Sleep Apneas, Obstructive” OR “Syndrome, Obstructive Sleep Apnea” OR “Syndrome, Sleep Apnea, Obstructive” OR “Syndrome, Upper Airway Resistance, Sleep Apnea” OR “Upper Airway Resistance Sleep Apnea Syndrome” AND “Bariatric Surgery” OR “Bariatric” OR “weight loss”.
Prospective observational studies and clinical trials were included, as well as retrospective studies with apneic patients undergoing weight loss treatment. No narrative reviews or systematic review articles were included in this review. The period covered the last 25 years, without age limitation, with subjects enrolled in the studies if undergoing weight loss treatment. The studies were evaluated by two investigators, who excluded the articles by title and abstract.
The following data were collected from the studies: title; author; year of publication; name of publishing journal; keywords; study design; age; sex; sample size; weight loss treatment; time of treatment for weight loss; interval for evaluation, average variation of weight loss in kilograms (kg) and/or Body Mass Index (BMI); variation of the Apnea-Hypopnea Index (AHI), or Respiratory Disorders Index (RDI) or Oxyhemoglobin Desaturation Index (ODI) and upper airway modifications.
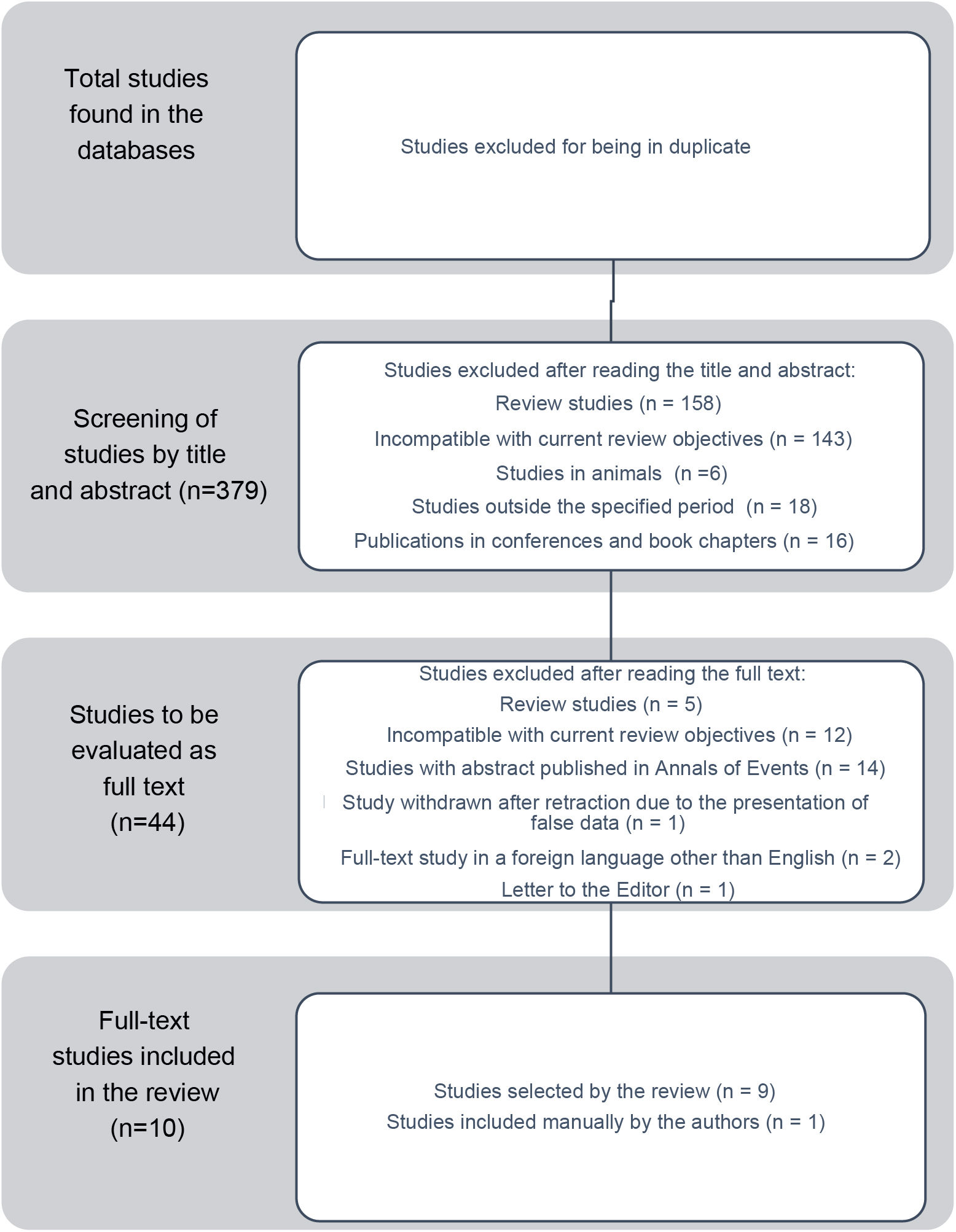
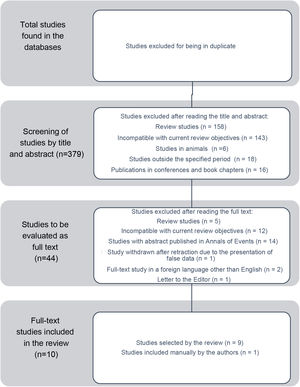
ResultsThe search in the databases was carried out until 08/23/2021 and resulted in 681 articles, as follows: Pubmed (title, subject and abstract) – 142; Web of Science (title, subject and abstract) – 160; Scopus (title, subject and abstract) - 189 and Embase (title, subject and abstract) – 190. After excluding articles in duplicate, 379 articles remained. A total of 337 articles were also excluded by the authors after reading the title and abstract (Fig. 1), totaling 44 studies to be reviewed by the authors. After searching for the full-text articles, only nine studies met the inclusion criteria (Fig. 1). After reading the 9 full text articles, another study that had not been mentioned in this review method, but met the research criteria, was included (Fig. 1).
The description of absolute measurements of the airways before and after weight loss were preferably extracted. When absolute variations were not available, predictors of variation in sleep apnea after weight loss were extracted. Data extracted from the articles included in the systematic review are summarized in Table 1.
General data of selected studies per year of publication.
| Author/year | Level of scientific evidence | n | Weight loss treatment | Evaluation interval | Airway evaluation method | Weight variation (kg or kg/m2) | Apnea Severity variation (AHI) | Airway alterations (p < 0.05) |
|---|---|---|---|---|---|---|---|---|
| K. Welch, G. Foster, C. Ritter at al.; 2002 | Series of cases: 4 | 9 | Behavioral treatment with a calorie-deficit diet of 1000–1500 kcal | 41–48 weeks | MRI (T1) | BMI: −6.1 ± 4.5 kg/m2, Weight: −17.1 ± 8.62 kg | Non -apneic (RDI < 1 before and after) | Airway volume (mm3) (retropalatal and retroglossal); Structural changes (Retropalatal: lateral walls); parapharyngeal fat deposits; Minimum retropalatal area (mm2); AP, pharyngeal lateral wall 26.8; Minimum retroglossal distance: latero-lateral, AP, lateral pharyngeal wall. |
| L. Busetto, G. Enzi, Inelmen, I. E., et al.; 2005 | Case-control: 3B | 17 | Intragastric balloon | 25 weeks (6 months) | Acoustic pharyngometry | BMI: −7.2 ± 9.9 kg/m2; Weight: −24.1 ± 14.5 kg | 59.3 ± 18.1–14.0 ± 12.4 (p < 0.01) | Orthostatic position: cross-sectional area of the pharynx at the level of the oropharyngeal transition, cross-sectional area of the pharynx. Supine position: cross-sectional area of the pharynx at the level of the oropharyngeal transition |
| Sutherland, K.; Lee, R.; Phillips, C, et al.; 2011 | Series of cases: 4 | 54 | sibutramine 10 or 15 mg daily +2500 kJ daily deficit diet with exercise advice | 25 weeks | CT | Weight: −7.8 ± 4.2 kg | 41.0 (27.2–56.3) to 26.1 (14.9–38.0) | Retropalatal volume; minimum velopharyngeal cross-sectional area, mean, latero-lateral diameter. Facial fat volume: mid-facial, lower facial, fat deposit. Hyoid to Posterior Nasal Spine (VA height) and Hyoid to C3. |
| Santos, M.; Laureano Filho, J.; Campello, R, et al.; 2011 | Series of cases: 4 | 17 | Roux-en-Y Gastric Bypass | 17 weeks | Cephalometry | BMI: −11.7 ± 5.04 kg/m2; Weight: −31.5 ± 14.9 kg | Not assessed | Facial type I: 9 vs. 10, II: 2 vs. 3, III 6 vs. 4. Angle between the nasion-sella and sella-C point lines (p < 0.001), Distance from the sella to the nasion (p = 0.038), Velopharyngeal space (p = 0.001), Distance from the hyoid bone to the chin (p = 0.042). predominance of Mallampati categories III and IV in the preoperative period and predominance of categories I and II in the postoperative period (p = 0.016). amygdala NS |
| Pahkala, R.; Seppä, J.; Ikonen, A, et al.; 2014 | RCT: 1B | 36 | Very low calorie diet + dietary advice + exercise recommendation vs. dietary advice + exercise recommendation | 1 year | CT | BMI intervention: −3.6 ± 3.0 kg/m2 (p < 0.001); control −1.6 ± 3.0 kg/m2 (p = 0.038) | Intervention: −10.0 ± 2.9 to −6.0 ± 4.8 (p < 0.001); control −9.7 ± 3.6 to −8.5 ± 7.6 (p = 0.061) | Fat deposit area (p = 0.003); control NS. |
| Naughton, M.; Monteith, B.; Manton, D. et al.; 2015 | RCT: 1B | 54 | Adjustable gastric banding surgery vs. diet | 24 months | Cephalometry | BMI: −6.7 ± 6,.5 kg/m2 (p < 0.001); Weight: −16.8 ± 23.8 kg (p < 0.001) | Intervention: 60.1 ± 27.0–40.4 ± 27.0 ev/h (p < 0.001); Control: 9.7–8.5 (p = 0.061) | Pre-measures as predictors for % AHI change: Mandibular body (menton ‒ gonion) was associated with % AHI change (r = 0.45; and in % weight change (r = 0.37; without adjustment for variables. Mandibular length also correlated with change in % AHI, Gn‒Go (r = 0.28). |
| Sutherland, K.; Phillips, C.; Yee, B. et al.; 2016 | Series of cases: 4 | 52 | Sibutramine 10 or 15 mg daily +2500 kJ daily deficit diet with exercise advice | 24 weeks | CT | BMI: −2.5 ± 2.9 (p < 0.001); Weight: −7.8 ± 12.8 (p < 0.001), variation −7.4% | 42.9 ± 21.3–26.8 ± 15.9 (p < 0,.001), variation −34.1% | Small, medium and large maxillomandibular volume (adjusted for height): small maxillomandibular volume (159.7–212.7 cm3) there was a strong correlation between weight loss and OSA improvement (rho = 0.65). |
| Al-Jumaily, A.; Ashaat, S.; Martin, B, et al.; 2018 | Series of cases: 4 | 10 | Roux-en-Y Gastric Bypass | 6M/12 M | CT | BMI: 6 months −48,.5 ± 6.5 to 33.7 ± 4.0 (p < 0.05); 12 months – 30.3 ± 3.6 | AHI: 6 months 38.1 ± 29.4 to 15.7 ± 15 (p < 0.05); 12 months 5.6 ± 10.2 (n = 6, p < 0.05) | Upper airway volume: 17,032 +/- 9691 to 17,749 +/- 6258 (6 months) and 18,262.3 ± 4256.6 mm3 (12 months). |
| Wang, S.; Keenan, B.; Wiemken, A, et al.; 2020 | Series of cases: 4 | 47 | Lifestyle change for weight loss: calorie restriction + increased physical activity + behavioral modifications (n = 49). Bariatric surgery (n = 18; sleeve gastrectomy (n = 8), Roux-en-Y gastric bypass; (n = 9), or gastric banding (n = 1) | 6M | MRI (T1) | BMI: −6.4 ± 5.8 kg/m2 (p < 0.001); Weight −18.6 ± 16.9 kg | AHI: 41.4 ± 27.6–18.6 ± 20.0 ev/h (p = 0.004) | Obtained from the supplement (n = 45). Variations of absolute measures. Retropalatal AP distance, minimum lateral distance. Soft tissue volumes: Total soft tissue, genioglossus, tongue fat, fat deposits, pterygoid, retropalatal lateral walls and total lateral walls. |
| Sutherland, K.; Chapman, J.; Cayanan, E, et al.; 2021 | Secondary review of 3 databases: 3B | 91 | Study 1: hypocaloric diet + lifestyle for 6 months (n = 58, 63.7% N). Study 2: maintenance diet for up to 12 months (n = 17, 18.7% N). Study 3 (n = 16, 17.6% N) bariatric surgery | 6‒12M | Craniofacial photography | BMI: −3.9 ± 5.1 (p < 0.001); Weight: −11.6 ± 15.4 | AHI: 36.2 ± 21.3–27.0 ± 20.4 (p < 0.001) | Obtained from the supplement. Mean maxillomandibular angle (°): 5.3 ± 2.4. Mean mandibular length (cm): 9.1 ± 1.2. 1st increase in maxillary-mandibular relationship angle predicts a decrease in AHI of 4.1% or 1.7 ev/h |
AHI, Apnea-Hypopnea Index; N, Number of study participants; F, Female; M, Male; M, Months; MRI, Magnetic Resonance Imaging; CT, Computed Tomography; BMI, Body Mass Index; RCT, Randomized Clinical Trial.
Following a chronological order of the examination methods used by the authors to assess the upper airway and grouping the studies to facilitate interpretation according to the examination and technique used, the main findings are summarized below.
In 2002, Kevin Welch,18 used Magnetic Resonance Imaging (MRI), the three-dimensional volumetric analysis technique, which allows objectively quantifying the volume of the tongue, soft palate, parapharyngeal fat deposits and pharyngeal lateral walls. A phantom investigation was performed to validate the technique and, subsequently, they demonstrated that the results were reliable and reproducible in normal individuals with no weight loss. After this validation, 12 obese and non-apneic women were studied before and after weight loss with behavioral changes and a calorie deficit diet (17.1 ± 8.62 kg [17.3%]). A significant increase was observed in the retropalatal areas (p < 0.05), as well as a reduction in the volumes of the retropalatal lateral walls and parapharyngeal fat deposits (p < 0.001), with no reduction in the tongue or soft palate volume.
With the technique validated by Welch,18 the group of researchers led by Dr. Richard Schwab, from the Division of Sleep Medicine at the University of Pennsylvania, published in 202017 a study describing the airway changes that occurred after weight loss in 67 obese apneic volunteers. Of the 67 volunteers, 47 achieved weight loss greater than 2.5% of the weight, considered clinically relevant. The obesity treatment included lifestyle changes (calorie-restricted diet associated with increased physical activity and behavioral changes, n = 49) or bariatric surgery in another 18 volunteers (sleeve method [n = 8], Roux-en-Y [n = 9], or gastric banding [n = 1]). With an average weight loss of 9.5% (from 123.4 to 111.0 kg, p < 0.001) in the entire sample, a reduction in the AHI of 38.5% (from 40.8 to 25.1 events/hour, p = 0.004) was observed. The group with clinically relevant weight loss showed a significantly increased pharyngeal lumen in the anteroposterior retropalatal diameter and an increase in the lateral minimum distance. As for changes in soft tissue volume, there was a significant reduction in the following measures: total soft tissue, genioglossus muscle, tongue fat, parapharyngeal fat deposits, pterygoid, retropalatal lateral walls and total lateral walls. The authors showed that reductions in tongue fat were strongly correlated with reductions in AHI (Pearson's rho = 0.62, p = 0.0001), even after controlling for weight loss (Pearson's rho = 0.36, p = 0.014).
Busseto19 categorized the pharyngeal cross-sectional area into three points (oropharyngeal junction cross-sectional area; mean pharyngeal cross-sectional area and glottic cross-sectional area) through acoustic pharyngometry. Evaluating 17 patients who completed the six-month treatment protocol with the intragastric balloon in extremely obese patients, with a mean initial BMI of 55.8 kg/m2, they observed a mean loss of 24.1 ± 14.5 kg; 14.5% weight loss with AHI reduction from 59.3 ± 18.1 to 14.0 ± 12.4 (p < 0.01). Significant changes were observed in the pharyngeal cross-sectional area at the level of the oropharyngeal transition, both, in orthostatic and in supine position; and an increase in the pharyngeal cross-sectional area in orthostatic position.
In 2011, Shutherland16 evaluated the airways of 54 obese, middle-aged men with moderate to severe OSA who underwent a 24-week sibutramine weight loss treatment, in which they achieved a mean loss of 7.8 kg. Using Computed Tomography (CT), they showed that the upper airway length and oropharyngeal or hypopharyngeal measurements did not change significantly in absolute measurements. The velopharyngeal volume increased from 5.3 cm3 to 6.3 cm3 (p < 0.01), with an increase in the minimum cross-sectional area of the velopharynx from 0.7 to 0.8 cm2 (p = 0.033), and mean area of the velopharynx from 1.4 to 1.7 cm2 (p = 0.002), and latero-lateral diameter from 1.5 to 1.7 cm (p = 0.009). Three-dimensional reconstructions of the fat volume were performed, showing a significant reduction in the volume of facial fat: in the middle and lower portions of the face and total fat deposits in the pharynx. The bone structure was also observed, especially the distance from the Hyoid to the Posterior Nasal Spine (upper airway height) and Hyoid to C3. In this study, the authors concluded that changes in the upper airway length seem to have a greater influence on the AHI reduction. Subsequently, in 2016,20 the same research group published a new assessment of the group of patients submitted to a six-month treatment with sibutramine, using CT images of the head to assess the size of the maxillomandibular skeletal framework around the upper airway region. In this evaluation, which classified the skeletal framework into three sizes (small, medium and large [adjusted for height)] the small maxillomandibular volume (159.7–212.7 cm3) had a greater correlation in OSA improvement with weight reduction (rho = 0.65; p = 0.004).
Pahkala et al., using CT images, evaluated the upper airway (UA) modifications in a randomized clinical trial,21 in which 36 patients underwent weight loss treatment for 12 months, with a low-calorie diet group accompanied by dietary advice and exercise recommendations (n = 19) versus dietary advice and exercise recommendations (n = 13). At the end of the 12 months, a 12.2% reduction in weight was observed, and there was a significant reduction in the area of parapharyngeal fat deposits after the intervention, from 194 to 158 mm2 (p = 0.003). In this study, there was a significant reduction in the AHI only in the group with supervised intervention, from 10.0 to 6.0 events/hour (p < 0.001), whereas the control group showed a decrease of 9.7 to 8.5 events/hour (p = 0.061).
A pilot study with 10 patients22 before and after bariatric surgery (Roux-en-Y), with apneic patients, evaluated individuals at 6 and 12 months postoperatively through three-dimensional reconstruction of tomographic sections. The reduction in AHI at 6 months was of 22.4 events/hour (p < 0.05) and at 12 months, evaluating 6 patients, 32.5 events/hour (p < 0.05). An increase in upper airway volume was observed, from 17,032 to 17,748.6 mm3 (6 months) and 18,262.3 mm3 (12 months; p < 0.05).
Santos23 used lateral cephalometry to assess craniofacial changes after bariatric surgery (Roux-en-Y), without assessing OSA. On average, the participants lost 31.5 kg, equivalent to a 25% reduction in baseline weight, but the study did not assess the presence or severity of obstructive sleep apnea. At a 17-week follow-up, changes were observed in facial type I: 9 vs. 10, II: 2 vs. 3, III: 6 vs. 4. There was a reduction in craniocervical length, with a reduction in the angle between the nasion-sella line and sella-C point line (angle between the base of the skull and the neck) from 116.02° to 113.86° (p < 0.001). There were also changes in the distance from the sella to the nasion: 67.12–67.36 mm (p = 0.038), increase in the retropalatal space from 10.86 to 14.49 mm (p = 0.001), reduction of the distance from the hyoid bone to the chin, 48.35 to 46.09 mm (p = 0.042), suggesting an approximation of the hyoid. Regarding the physical examination, the authors point out a predominance of Mallampati categories III and IV in the preoperative period and a predominance of categories I and II in the postoperative period (p = 0.016).
Lateral cephalometry was also used in the initial evaluation of 57 obese apneic patients who participated in a randomized trial24 with dietary treatment versus bariatric surgery (adjustable gastric banding). With the intention of evaluating predictors of AHI improvement after two years of weight loss, the authors observed that the mandibular body was longer (Me‒Go [r = 0.45; p = 0.001]), and the mandibular length (Gn‒Go [r = 0.28; p = 0.034]) was also associated with the greatest change in the AHI. Regarding the group average, there was a 12.2% reduction in weight and a 24.3% reduction in AHI after two years.
Exploring how facial morphology could correlate with AHI changes and the efficiency of weight loss as a treatment for OSA, Sutherland25 combined data from three studies that performed craniofacial photographs before different weight loss treatments. This meta-analysis consisted of 91 participants, with a mean weight loss of 11.6 kg between six and 12 months of follow-up, with a mean reduction in AHI of 9.2 events/hour and an increase in the maxillary-mandibular relationship (mean maxillomandibular angle of 5.3°) predicting a decrease in AHI of 4.1% or 1.7 event/hour at each degree.
DiscussionWhen evaluating the results of the anatomical site, considering the pre- and post-weight loss changes described in eight of the studies included in the review, six16–19,22,23 studies indicate an increase in the area of the retropalatal region. Three of these studies showed this increase occurring in the lateral region of the pharynx, with a reduction in the volume of the lateral pharyngeal walls.17,18 A significant reduction of parapharyngeal fat deposits was described in studies with three-dimensional reconstruction.16–18,21 Wang,17 using MRI, observed fat reduction on the lateral walls and tongue, and volumetric reduction of all soft tissues of the pharynx, pterygoid and genioglossus muscles. Two studies, one using CT images16 and other using lateral cephalometry, showed reduction in the airway height, identifying an approximation of the hyoid to the posterior nasal spine and reduction of the distance between the hyoid and chin.
The increase in airway collapsibility caused by an increase in soft tissue due to excess weight is described as one of the main pathophysiological links between OSA and obesity.26 However, although weight reduction is one of the most frequently mentioned therapeutic measures in the treatment of OSA, and its effectiveness has been previously endorsed by studies with a high level of evidence,9,10,25 few studies have evaluated the impact of this treatment on the upper airways.
The quality and level of scientific evidence of the studies included in this review was regular: only six studies aimed to describe upper airway modifications after weight loss, one was a randomized clinical trial,21 the other five were prospective observational studies; thus, the majority of studies included in this review had a “low” or “very low” level of evidence.27 Two studies24,25 did not include data on the airways after weight loss; however, the description of coincident predictive findings, of mandibular length and maxillomandibular angle correlated with OSA improvement were considered relevant. The studies included in this review had a limited number of participants not allowing a generalization of the results to all obese patients who undergo weight loss, but they still constitute the current scientific evidence that seeks to describe how weight loss modifies the anatomy of the upper airways, thus having an impact on OSA.
ConclusionCurrent studies show that significant weight loss increases the airways of obese individuals, with a reduction in the volume of parapharyngeal structures, notably at the retropalatal site, where there is an apparent gain in the lateral area of the airway. These changes were already expected and corroborate the improvement of OSA that occurred in these studies. Another change that corroborates the improvement in apnea severity is the movement of the hyoid, reducing the height of the airway, which could allow greater caudal traction and distension of the pharyngeal tissues during the respiratory cycle.
Given the limited number of studies and their level of scientific evidence, the authors conclude that studies with less bias regarding the heterogeneity of treatment methods for weight loss and with a greater number of participants are necessary to elucidate the upper airway modifications after weight loss and how these changes can impact OSA.
Sources of fundingThe present study did not receive financial support from funding agencies or companies. We declare that the author Michel Cahali is an investor in the company Biologix, a Brazilian company that manufactures a home polygraph device sold in Brazil. This device was not used in this research and the conflict is only lateral, as this is a study in the area of sleep.
Conflicts of interestThe authors declare no conflicts of interest.
Peer Review under the responsibility of Associação Brasileira de Otorrinolaringologia e Cirurgia Cérvico-Facial.