Tumors of the lip and oral cavity differ in various aspects; therefore a clarification of the distinctions among these sites may help to better understand the biologic behavior of neoplasms occurring in these locations.
ObjectiveConsidering that angiogenesis and lymphangiogenesis are two major elements that can influence various aspects of tumor biology, we aimed to compare these factors between squamous cell carcinoma of the lower lip and oral cavity.
MethodsA total of 84 primary squamous cell carcinomas including 45 oral and 39 lower lip tumors were selected and immunohistochemically stained with monoclonal antibody against D2-40 and CD105. Mean microvessel density was assessed in tumoral tissue, while lymphatic vessel density was calculated in both neoplastic tissue and invasion front. Data were statistically analyzed using t-test and p-values of <0.05 were considered significant.
ResultsWe found a mean microvessel density±standard deviation of 31.94±18.9 in oral cavity and 27.54±20.8 in lower lip squamous cell carcinomas, with no significant difference (p=0.32). Mean lymphatic vessel density±standard deviation was 13.05±8.2 and 16.57±10.79 in of oral cavity and lower lip neoplastic tissue, respectively. The corresponding values were 9.94±5.59 and 12.50±7.8 in the invasive front. Significant differences were not observed in either of the lymphatic vessel density variables between the two sites.
ConclusionAccording to our results, it seems that the search for additional factors other than those related to the vasculature should continue, to help clarify the differences in biologic behavior between lower lip and oral cavity squamous cell carcinomas.
Os tumores de lábio e da cavidade oral diferem em vários aspectos; portanto, o conhecimento das diferenças entre eles pode ajudar na melhor compreensão do comportamento biológico das neoplasias que ocorrem nesses locais.
ObjetivoConsiderando que a angiogênese e a linfangiogênese são dois elementos importantes que podem influenciar diversos aspectos da biologia dos tumores, objetivamos comparar esses fatores entre o carcinoma de células escamosas (CCE) de lábio inferior e da cavidade oral.
MétodoNo total, foram selecionados 84 CCEs primários (45 tumores da cavidade oral e 39 tumores de lábio). Esses tumores foram corados por processo imuno-histoquímico com anticorpo monoclonal anti-D2-40 e CD105. Avaliamos a densidade média de microvasos (DMV) no tecido tumoral, enquanto que a densidade vascular linfática (DVL) foi calculada tanto no tecido neoplásico como no front de invasão. Os dados foram estatisticamente analisados com o uso do teste t e valores de p<0,05 foram considerados significantes.
ResultadosChegamos a uma média para DMV±DP de 31,94±18,9 para CCEs na cavidade oral e de 27,54±20,8 no lábio inferior, sem diferença significante (p=0,32). As médias para DVL±DP foram de 13,05±8,2 e 16,57±10,79 no tecido neoplásico da cavidade oral e lábio inferior, respectivamente. Os valores correspondentes foram 9,94±5,59 e 12,50±7,8 no front invasivo. Não foram observadas diferenças significantes nas duas variáveis DVL entre os dois locais.
ConclusãoDe acordo com os nossos resultados, a pesquisa por fatores adicionais, além daqueles relacionados à vasculatura, deve ter continuidade, para auxiliar no esclarecimento das diferenças do comportamento biológico entre CCEs no lábio inferior e na cavidade oral.
Squamous cell carcinomas (SCCs) originate from epithelial cells of various organs and their biologic behavior depends on different factors, one of which is the anatomic location of the tumor.1 A good example of this fact is the considerable etiologic and prognostic differences between SCCs of the lip and oral cavity, with lip neoplasms demonstrating a lower tendency toward regional lymph-node metastasis and a higher survival rate of approximately 90%.2,3
Many factors are involved in the etiopathogenesis of SCC. Contrary to SCC of the oral cavity where tobacco use is the most well-known etiologic factor, chronic exposure to sunlight has been suggested as an important element in SCC of the lower lip, which is known to receive more ultraviolet radiation than the upper lip.2,4,5 Recent studies have shown that the expression of some markers related to tumor microenvironment and neoplastic cells of lip SCCs are different from those of the oral cavity.2,3 Therefore, it seems that the differences between these sites are not limited to etiology and prognosis, but may also be related to molecular factors associated with their stroma and cellular structures.2,3 Consequently, a number of investigators believe that SCC of the lip should be regarded as a separate entity and be evaluated as such. On the other hand, some cellular-molecular studies on these locations have not shown any biological difference in the evaluated markers.6,7
Angiogenesis is an important and fundamental process in the progression and metastasis of malignancies. Before 1960, researchers believed that nutrition and blood supply of neoplastic tissues were simply provided through dilation of blood vessels available in the tumor. Subsequent studies revealed that angiogenesis, the formation of new blood vessels, is vital to the growth and propagation of malignancies.8 Development of a network of new blood vessels in the tumor is essential to provide nutrients and oxygen and remove waste products. For the initiation of angiogenesis, various molecules are released from malignant cells, which send signals to the surrounding host tissues. This may result in the activation of certain genes, followed by protein production, leading to the induction of angiogenesis.9,10
Lymphangiogenesis is the formation of new lymphatic vessels from pre-existing vasculature and similar to angiogenesis has several induction mechanisms.11 The growth of lymphatic vessels occurs in a variety of normal and pathologic processes like wound healing, inflammation, and progression of malignancies.12,13
SCC of the oral cavity and lip have been separately evaluated in terms of angiogenesis and lymphangiogenesis, and various reports exist on the association of these processes with the prognosis and invasion of SCC.14–16 However, a limited number of studies with conflicting results have compared angiogenesis and lymphangiogenesis between these sites.17,18 It is noteworthy that in these investigations, SCC of both upper and lower lips have been grouped together and evaluated as a single entity. Considering that the lower lip SCC has not been exclusively evaluated in comparison with SCC of the oral cavity and the important differences between upper and lower lip tumors,19 we aimed to compare angiogenesis and lymphangiogenesis between lower lip and oral cavity SCC using CD105 and D2-40 markers.
MethodsSamplesThis retrospective study was performed on individuals with primary SCC who were consecutively visited at the Cancer Institute of Imam Khomeini Hospital Complex, affiliated with Tehran University of Medical Sciences between 2007 and 2012, using the patient record archive of this Center. Cases which had a history of chemotherapy, radiotherapy, or any other treatment prior to surgery were not included in this work. Excisional biopsy samples with significant necrosis and inadequate tissue were also excluded from the study. Age and sex were recorded for each subject according to the clinical data provided in their medical charts. Formalin-fixed paraffin blocks of all lesions corresponding to the patient charts of the selected cases were retrieved from the pathology archive to be used for immunohistochemical analysis. This project was approved by the ethics committee of Tehran University of Medical Sciences (code n° 70-10646).
Immunohistochemical stainingFormalin-fixed paraffin-embedded tissue sections (3μm) were mounted on poly-l-lysine-coated slides and subjected to deparaffinization in xylene, followed by rehydration in graded alcohol and antigen retrieval. For D2-40, this was done by immersing the specimens in citrate buffer (0.1M, pH 6) and heating in a microwave oven for 2 cycles of 15min each, and for CD105 pretreatment with proteinase K was performed for 5min. Endogenous peroxidase was then blocked by incubating the sections in a solution of 3% hydrogen peroxide and methanol for half an hour. After washing with Tris-buffered saline (TBS), the specimens were treated with either D2-40 (D2-40, Dako Cytomation) or CD105 (SN6h, Dako, Glostrup, Denmark) monoclonal antibodies for 1h in a humid chamber at 1:1000 and 1:30 dilutions, respectively. TBS was used for rinsing before incubating with EnVision System (Dako Cytomation, Glostrup, Denmark) at room temperature for 30min. Antigen–antibody reaction was visualized with diaminobenzidine, and counterstaining was carried out with Mayer's hematoxylin. Positive controls consisting of breast carcinoma with known immunoreactions for D2-40 and normal liver tissue for CD105 along with negative controls (omission of primary antibody for negative control) were run simultaneously with the experimental slides.
Staining evaluationMicrovessel density (MVD) and lymphatic vessel density (LVD) were quantified according to the method described previously.20 In brief, using a double-headed microscope (Olympus BH2, Tokyo, Japan), five hotspots were selected at 100× magnification by two oral pathologists, followed by microvessel counting at 400× (field size: 0.18mm2) and calculating the mean microvessel count for each sample. Any possible disagreements were resolved by consensus.
Statistical analysisStatistical analysis was performed using t-test, and p<0.05 was considered significant.
ResultsWe obtained a total of 84 cases of SCC, 45 of which were located in the oral cavity and 39 in the lower lip. Of the 39 lower lip SCC samples, 5 (13.5%) were female and 34 (86.5%) were male, corresponding to 31 (68.9%) male and 14 (31.1%) female patients with SCC of the oral cavity. The age range of the patients with lower lip and oral cavity SCC was 31–90 (mean: 65) and 19–64 (mean: 61) years, respectively. Oral cavity tumors were located in the tongue (21: 46.7%), floor of the mouth (8: 17.8%), buccal mucosa (6: 13.3%), gingiva (6: 13.3%) and maxilla (4: 8.9%).
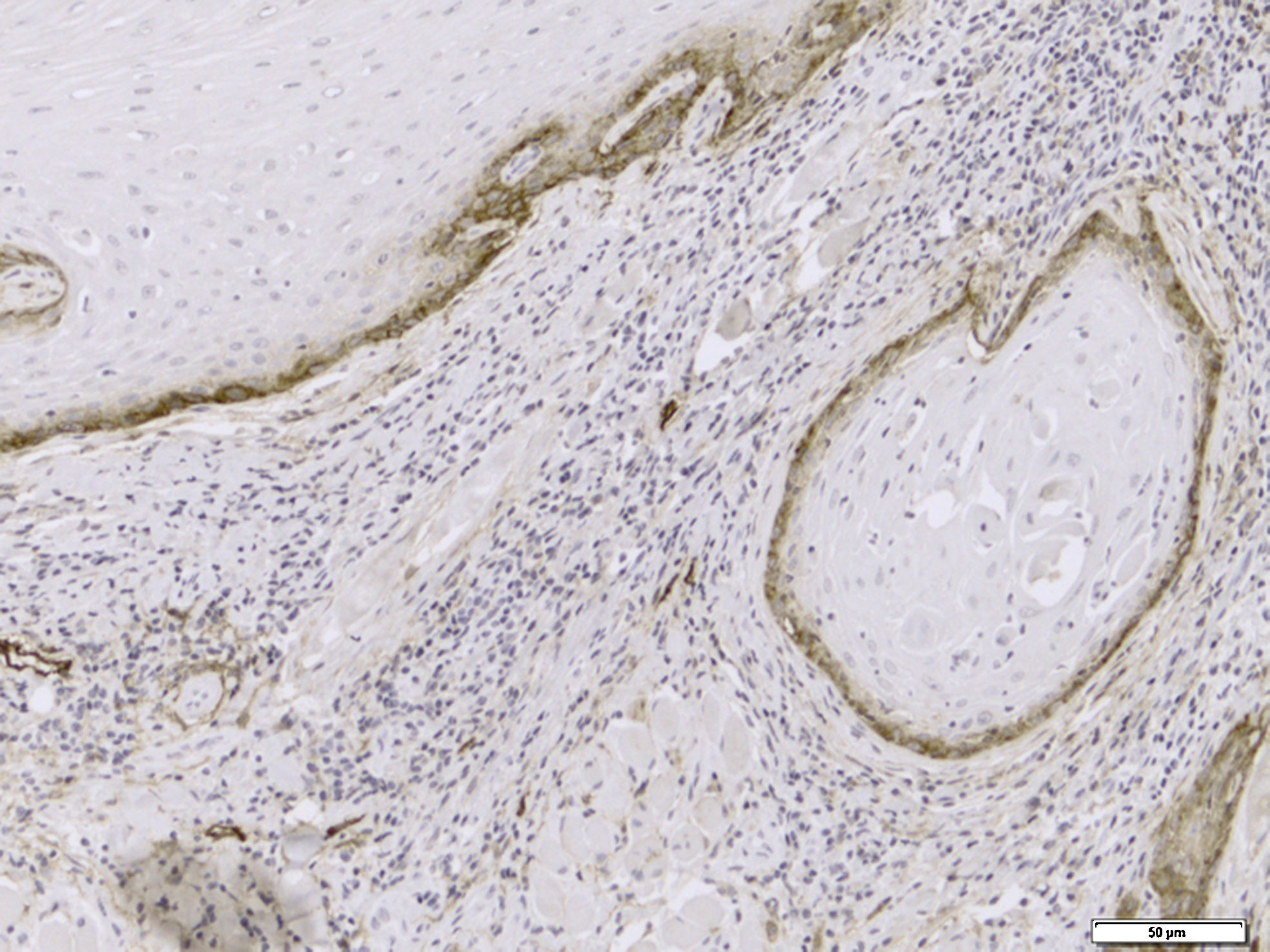
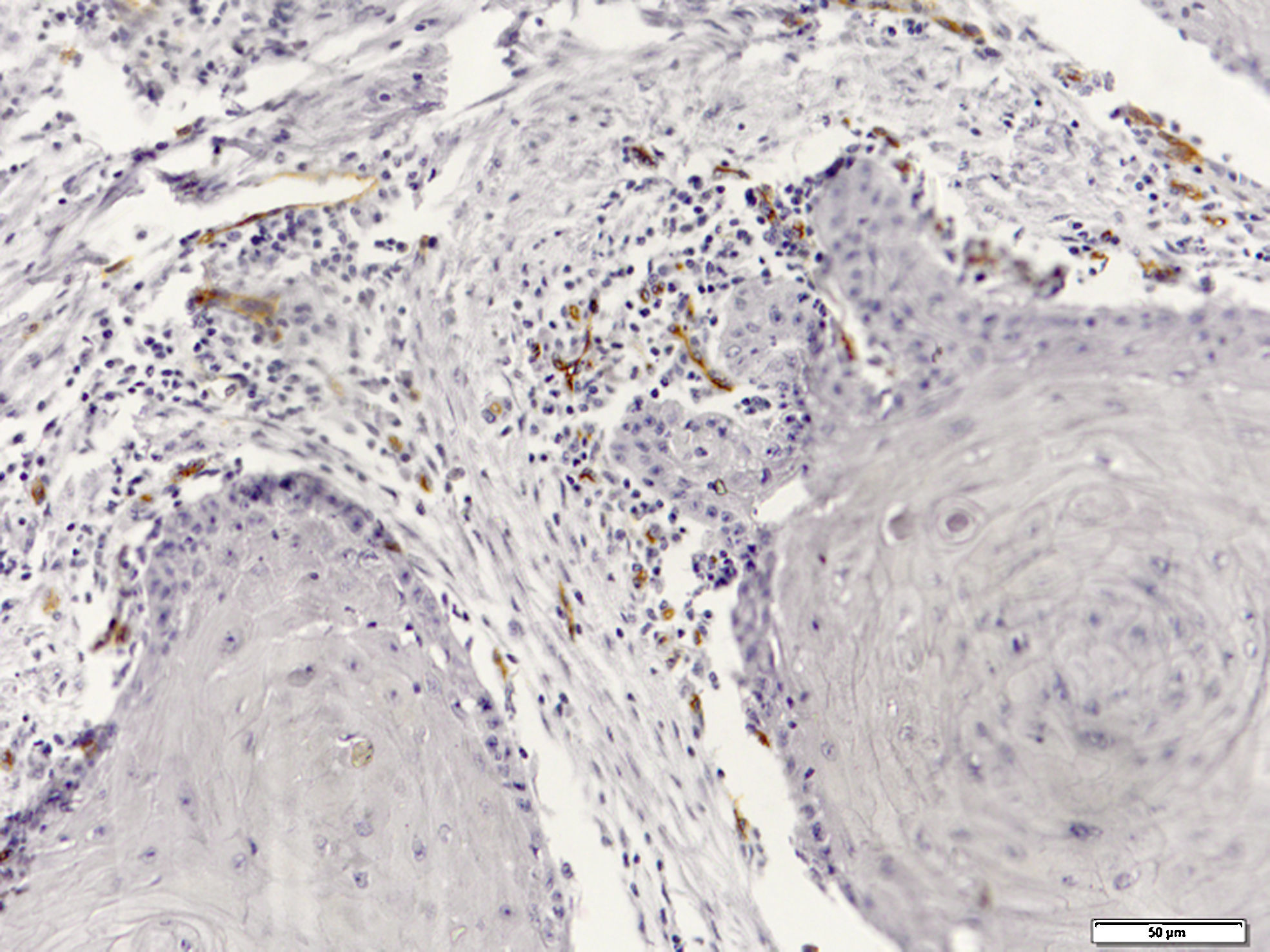
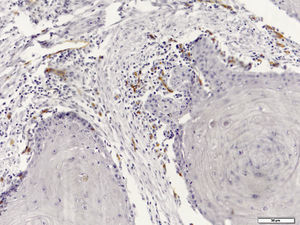
Immunohistochemical evaluation of LVD was performed separately in the neoplastic tissue and tumor invasive front (Fig. 1), while MVD was assessed generally in the SCC tissues (Fig. 2).
Mean MVD±SD was 31.94±18.9 in the oral cavity and 27.54±20.8 in the lower lip. The highest and lowest mean MVD values (92.00 and 5.30, respectively) were observed in the oral cavity SCC group. No significant difference was found between the lower lip and oral cavity (p=0.32)
Mean LVD±SD in the tumoral tissue was 13.05±8.2 in the oral cavity and 16.57±10.79 in the lower lip SCC group. Tumor invasive margin demonstrated a mean LVD±SD of 9.94±5.59 and 12.50±7.8 in the oral cavity and lower lip, respectively. The highest and the lowest amounts of mean LVD were observed in the tumor front when compared with the neoplastic tissue, with the highest value being 48.67 in an oral cavity tumor and the lowest counting 0.67 in the lower lip. Neither tumoral tissue (p=0.105) nor invasive front (p=0.098) showed significant differences between the lower lip and oral cavity.
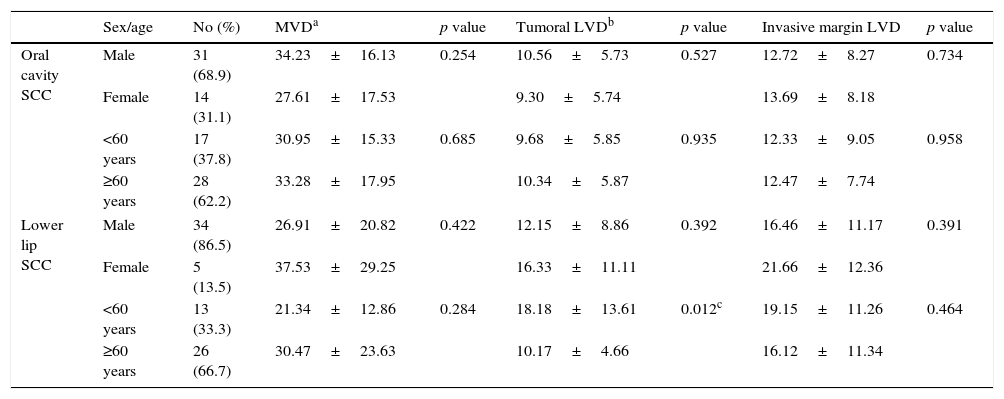
Comparison of MVD and LVD between oral cavity and lower lip SCCs according to age and sex is shown in Table 1. Based on our results, lower lip SCC patients younger than 60 years of age demonstrated significantly higher neoplastic LVD compared to those older than 60 years (p=0.012).
Comparison of microvessel- and lymphatic vessel-density in oral cavity and lower lip SCC.
| Sex/age | No (%) | MVDa | p value | Tumoral LVDb | p value | Invasive margin LVD | p value | |
|---|---|---|---|---|---|---|---|---|
| Oral cavity SCC | Male | 31 (68.9) | 34.23±16.13 | 0.254 | 10.56±5.73 | 0.527 | 12.72±8.27 | 0.734 |
| Female | 14 (31.1) | 27.61±17.53 | 9.30±5.74 | 13.69±8.18 | ||||
| <60 years | 17 (37.8) | 30.95±15.33 | 0.685 | 9.68±5.85 | 0.935 | 12.33±9.05 | 0.958 | |
| ≥60 years | 28 (62.2) | 33.28±17.95 | 10.34±5.87 | 12.47±7.74 | ||||
| Lower lip SCC | Male | 34 (86.5) | 26.91±20.82 | 0.422 | 12.15±8.86 | 0.392 | 16.46±11.17 | 0.391 |
| Female | 5 (13.5) | 37.53±29.25 | 16.33±11.11 | 21.66±12.36 | ||||
| <60 years | 13 (33.3) | 21.34±12.86 | 0.284 | 18.18±13.61 | 0.012c | 19.15±11.26 | 0.464 | |
| ≥60 years | 26 (66.7) | 30.47±23.63 | 10.17±4.66 | 16.12±11.34 | ||||
Metastasis of malignant cells to lymph nodes is one of the major prognostic factors in many solid tumors like oral SCC.21 Angiogenesis and lymphangiogenesis provide new vessels through which malignant cells can leave the surrounding area of the primary tumor.13 Different aspects of these two processes have been evaluated in SCC of the oral cavity and lip using different markers.15–18 CD105 is currently employed for the evaluation of newly formed vessels. This protein preferably binds to the active endothelial cells involved in the process of angiogenesis. For this reason, CD105 is highly expressed in proliferating endothelial cells, while its expression is weak or negative in normal vessels. The power and ability of CD105 for quantitative differentiation between active/proliferating and normal/quiescent endothelial cells makes it possible to evaluate newly formed tumor blood vessels more accurately.22,23
On the contrary, fewer studies have been performed on lymphatic vessels because of an absence of appropriate ocular techniques. In the past, many researchers believed that tumors, due to the lack of lymphatic vessels, cannot induce lymphangiogenesis. In the recent decade, specific antibodies against lymphatic endothelial cells have been identified, leading to a modification of the general viewpoint toward this process.11 D2-40 is a marker that is expressed on endothelial cells of lymphatic vessels and has been used for the evaluation of LVD in recent years.24 In the present study we used D2-40 and CD105 to evaluate angiogenesis and lymphangiogenesis in SCC of the lower lip and oral cavity. Our results showed higher MVD in the oral cavity compared to the lip, but the difference was not significant. In agreement with our findings, Mărgăritescu et al.,25 using CD-105, reported no significant difference between these sites; however, they found MVD to be higher in lip SCCs. In contrast, Oliveira-Neto et al.17 demonstrated significant differences in MVD between SCCs of the lip and oral cavity, with MVD being higher in oral tumors. Chronic sun exposure, as seen in photoaged skin, has been suggested to decrease the number of blood vessels in the upper dermis,26 while oral cavity mucosa is known for its high vascularity and efficient blood supply. This fact may be responsible for the higher MVD of our intraoral SCCs. In addition, considering the metastasis-promoting role of angiogenesis, the higher MVD of oral cavity tumors in this study is in line with previous reports, stating that oral cavity tumors are more prone to lymph-node metastasis and demonstrate a lower survival rate when compared to lip tumors.2,3
Contrary to our MVD findings, the mean LVD in the current investigation was higher in lower lip versus oral cavity SCCs; however, like MVD, no significant difference was observed between the sites in neither tumoral tissue nor invasive margin. Oliveira-Neto et al.17 also demonstrated that the difference in LVD between the oral cavity and lower lip was not significant; however, their results showed a slightly higher LVD in lip SCCs compared to oral cavity tumors. In contrast to our findings, Watanabe et al.,18 in a sample of 105 oral cavity SCCs and only three cases of lip tumors, reported significantly higher mean LVD in lip versus oral cavity SCCs. Since metastasis to lymph nodes of SCC is more common in the oral cavity than the lower lip and lymphatic vessels provide easier access to the lymphatic system for malignant cells, the mean LVD was expected to be higher in oral cavity SCCs compared to lip tumors, while the opposite was observed in our study. Different cellular and molecular factors are involved in the development and progression of lymphatic metastasis, of which lymphangiogenesis is only one of them. Evaluation of other effective factors may better reveal the biologic differences between lower lip and oral cavity SCC.
The statistically insignificant differences in both factors between the lip and oral cavity observed in the current investigation are comparable to previous studies, which found lip cancer to be closely related to upper digestive tract malignancies.5 Additionally, it is noteworthy that in all of the abovementioned studies, “lip specimens” included the upper and lower lips, while we excluded upper lip SCCs from our study sample. Consequently, our findings reflect MVD and LVD of lower lip tumors in comparison to oral neoplasms, and our results therefore may not be accurately compared with those investigations. The importance of the exclusive selection of lower lip SCC is reflected in the fact that they have been shown to be biologically distinct from upper lip tumors. Malignancies in these locations also differ in prevalence, and possibly in etiology: SCC of the lower lip is more common than in the upper lip, and the role of UV light and pipe smoking is more prominent in causing lower lip versus upper lip SCC.19,27 On the other hand, the growth of lower lip malignancy is slower than its counterpart, with a better prognosis. For these reasons, some investigators suggest that SCC of the upper lip should be evaluated as a separate entity in order to render more reliable results.26–29 Regarding demographic data, we found a significantly higher neoplastic LVD in patients with lower lip SCCs who were younger than 60 years, as compared to those who were aged 60 or older. This is in accordance with previous studies demonstrating an aggressive course of disease in some young patients and those indicating differences in the molecular profile of young and old patients with SCC.30 It should be mentioned that if we had access to TNM staging, survival and metastasis data of the patients, we could comment on the relationship of metastasis with angiogenesis and lymphangiogenesis in lower lip and oral cavity SCC with more certainty.
Final commentsIn recent years, studies performed on a number of cellular and molecular markers in lip and oral cavity SCCs have revealed some differences, indicating their biologic variations.2,3 On the other hand, the expression of other proteins showed no difference between these two groups.7,8 Based on the results of the current investigation, angiogenesis and lymphangiogenesis do not seem to be helpful in clarifying the biologic difference of lower lip and oral cavity SCC. It seems that the search for additional factors other than those related to the vasculature should continue to help clarify the differences in biologic behavior between lower lip and oral cavity SCCs.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Alaeddini M, Etemad-Moghadam S. Lymphangiogenesis and angiogenesis in oral cavity and lower lip squamous cell carcinoma. Braz J Otorhinolaryngol. 2016;82:385–90.