There is a controversy concerning the terminology and definition of rhinitis in pregnancy. Gestational rhinitis is a relatively common condition, which has drawn increasing interest in recent years due to a possible association with maternal obstructive sleep apnea syndrome (OSAS) and unfavorable fetal outcomes.
ObjectiveTo review the current knowledge on gestacional rhinitis, and to assess its evidence.
MethodsStructured literature search.
ResultsGestational rhinitis and rhinitis “during pregnancy” are somewhat similar conditions regarding their physiopathology and treatment, but differ regarding definition and prognosis. Hormonal changes have a presumed etiological role, but knowledge about the physiopathology of gestational rhinitis is still lacking. Management of rhinitis during pregnancy focuses on the minimal intervention required for symptom relief.
ConclusionAs it has a great impact on maternal quality of life, both the otorhinolaryngologist and the obstetrician must be careful concerning the early diagnosis and treatment of gestational rhinitis, considering the safety of treatment measures and drugs and their current level of evidence.
Há grande confusão quanto à terminologia e definição da rinite na gestação. A rinite gestacional é uma condição relativamente comum que vem ganhando importância nos últimos anos pela descoberta de sua associação com a SAOS materna e possíveis desfechos desfavoráveis ao feto. Há pouca evidência na literatura nacional sobre o tema.
ObjetivoRevisar o conhecimento científico atual sobre a rinite na gestação e suas evidências disponíveis.
MétodoRevisão de literatura estruturada.
ResultadosA rinite gestacional e a rinite “durante a gestação” são condições com alguns pontos de fisiopatologia e tratamento semelhantes, mas com definições e prognósticos diferentes. O papel dos hormônios nessas condições vem sendo sugerido por muitos trabalhos, mas o conhecimento sobre a fisiopatogenia da rinite gestacional ainda é escasso. O manejo da rinite na gestação requer o mínimo de intervenção com o maior alívio sintomático possível.
ConclusãoDado o grande impacto na qualidade de vida da gestante, tanto o otorrinolaringologista quanto o obstetra devem estar atentos para o diagnóstico precoce e manejo desta entidade, considerando o perfil de segurança e o nível de evidência das medidas e medicamentos disponíveis atualmente.
Gestational rhinitis is a relatively common condition, but is seldom discussed in the national literature. It has gained importance in recent years, mainly due to the discovery of its association with snoring and obstructive sleep apnea syndrome (OSAS) during pregnancy, and indirectly with preeclampsia, a leading cause of maternal morbidity and mortality.1 Additionally, studies have shown its association with gestational hypertension, intrauterine growth retardation, and lower Apgar scores in neonates.1,2
The first studies that associated the nasal obstruction symptom with female hormones appeared in the late nineteenth century.1 In 1884, MacKenzie3 presented a series of observations on the increased volume of the inferior turbinates during menstruation and sexual stimulation, expanding his theories to pregnancy in 1898.4 However, it was only in 1943 that Mohun5 presented a series of cases on this entity that would be the precursor of gestational rhinitis, terming it “vasomotor rhinitis of pregnancy.” Nasal symptoms appear between the third and seventh months of pregnancy and usually normalize within ten days after delivery.5
Gestational rhinitis terminology still creates much confusion. Some authors emphasize the importance of differentiating rhinitis “during pregnancy” from gestational rhinitis (or pregnancy-induced rhinitis). Rhinitis during pregnancy is an entity that includes all types of rhinitis (allergic, drug, non-allergic, with vasomotor component, among others), which would be present before, during, and after pregnancy by definition.6 Gestational rhinitis, in turn, is defined as nasal obstruction that is not present before pregnancy, typically occurs in the second or third trimesters, with a duration equal to or greater than six weeks, shows no allergic cause or upper airway infection signs and symptoms resolve completely within two weeks after delivery.1,7,8
The aim of this study was to conduct a literature review on gestational rhinitis and extend some concepts with a comparative basis (such as physiopathology and treatment) for other types of rhinitis in pregnancy.
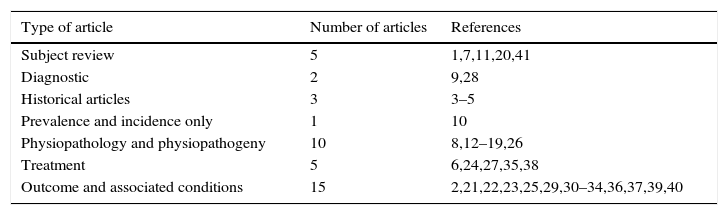
MethodsA literature review was carried out in the PubMed, MEDLINE, and SciELO databases using the terms “rhinitis and pregnancy”. Of the 506 initially listed articles, two researchers independently selected 66 and 59 of them, respectively, using as selection criteria articles that had gestational rhinitis or other types of rhinitis during pregnancy as the main topic. Subsequently, 40 common articles were chosen from the two selections, including the most relevant regarding the criteria of definition, diagnosis, etiology, etiopathogenesis, differential diagnosis, and treatment of both gestational rhinitis and rhinitis during pregnancy. The distribution of articles found according to topic is shown in Table 1.
Type of articles found and correspondence in the references.
| Type of article | Number of articles | References |
|---|---|---|
| Subject review | 5 | 1,7,11,20,41 |
| Diagnostic | 2 | 9,28 |
| Historical articles | 3 | 3–5 |
| Prevalence and incidence only | 1 | 10 |
| Physiopathology and physiopathogeny | 10 | 8,12–19,26 |
| Treatment | 5 | 6,24,27,35,38 |
| Outcome and associated conditions | 15 | 2,21,22,23,25,29,30–34,36,37,39,40 |
Approximately 20%–40% of women report rhinitis symptoms during childhood or adolescence, and 10%–30% of these women report symptom worsening during pregnancy.9 Population studies with relatively small samples often do not differentiate gestational rhinitis from other types of rhinitis, showing a prevalence ranging between 18% and 30% (for all types of rhinitis during pregnancy).7 The largest population study on prevalence was performed in Sweden through questionnaires and included 599 patients, excluding those who already had signs of rhinitis before pregnancy, revealing a prevalence of gestational rhinitis of 22%.10
Another recent study, however, assessed 109 pregnant women through questionnaires and anterior rhinoscopy and reached a prevalence of 9% for gestational rhinitis, which was consistent with other studies.11 There were no Brazilian data on gestational rhinitis prevalence. This prevalence variation is due not only to the difficulty of having access to and diagnosing the disease and the need for a strict criterion, but also the fact that rhinitis and asthma may worsen, improve, or remain unchanged throughout the course of pregnancy, as well as the fact that they may vary in terms of clinical presentation throughout the gestational weeks and according to each patient's genetic susceptibility.9
Etiology and physiopathogenyAlthough many etiological factors have been proposed, the current knowledge about the physiopathology of gestational rhinitis is still scarce.7,9 It is presupposed that the placental trophoblastic hormone can stimulate hypertrophy of the nasal mucosa during pregnancy.9 Moreover, estrogen may contribute to this effect by increasing histamine receptors in epithelial cells and the microvasculature.12 Progesterone may also play a role by optimizing local vasodilation in the nose by increasing the circulating blood volume that occurs physiologically during pregnancy.13
Nasal physiology studies have shown significant alterations in anterior rhinoscopy, rhinomanometry, and specific rhinitis questionnaire scores compatible with decreased patency of air passage through the nasal cavity during the course of pregnancy.14 However, all these data – including the role of hormones–are conflicting, as there have been studies demonstrating nasal obstruction improvement during the course of pregnancy in a significant number of patients.15
Regarding risk factors for the development of gestational rhinitis, smoking was the only one identified with significant evidence.7,16 The same study found that the specific IgE to house dust mites was a predisposing factor for the development of the disease.16 There is no association between gestational rhinitis and pre-existing asthma.8 Also, no association was observed with maternal age, gestational age, child's gender, and parity.1,7,17 In relation to the actual allergic rhinitis, it was observed that the electron microscopy findings in pregnant women with nasal symptoms were identical to those with allergic rhinitis.18
Considering this and the fact that a considerable proportion of patients with gestational rhinitis have sensitivity to house dust mites, it was suggested that patients with gestational rhinitis represent an allergic rhinitis subgroup, albeit with spontaneous resolution after birth, according to the definition.7 Additionally, serum markers for allergic disease such as soluble intercellular adhesion molecule-1 (sICAM-1) are not elevated in gestational rhinitis.7
Diagnosis and clinical significanceGestational rhinitis is characterized by nasal obstruction in the last six or more weeks of gestation, with complete resolution within two weeks after delivery.1,7,8 The diagnosis is clinical and can be suspected only by the worsening of nasal obstruction symptoms (which were not present previously) in pregnant patients, and is not secondary to other conditions – the differential diagnosis includes sinusitis, allergic rhinitis, drug-induced rhinitis, acute or subacute upper airway infection, and pregnancy granuloma.1,9
It is important to be cautious regarding the nasal obstruction criteria in pregnant women, considering as positive only a worsening pattern or a symptom that has significant impact on patient quality of life, as this is an alteration that is part of the normal physiology of pregnancy.19 In addition to the obstruction, patients with gestational rhinitis often present with rhinorrhea.1
Allergic rhinitis is usually an existing clinical picture that can also occur during pregnancy. Unlike gestational rhinitis, in which nasal obstruction is the main symptom, patients with allergic rhinitis during pregnancy have rhinorrhea, pruritus, sneezing, as well as nasal congestion.9
The nasal obstruction caused by rhinitis – either gestational or not – may be associated with a worsening of the pregnant woman's quality of sleep, in addition to snoring and obstructive sleep apnea (OSA), although the latter may be the result of a combination of factors, involving weight gain during pregnancy.20
Oral breathing caused by nasal obstruction worsening during gestational rhinitis may lead to a decrease in inhalation of nitric oxide (NO) – produced mainly in the maxillary sinuses – to the lung, which decreases vascular resistance, in addition to improving local oxygenation.1,7 The reduction in pulmonary NO inhalation can have a deleterious effect on the fetus, leading to maternal hypertension, intrauterine growth retardation, preeclampsia, and lower Apgar scores of newborns.1,2 Additionally, nasal obstruction, with quality of sleep impairment, can lead to the abuse of topical nasal decongestants, leading to an associated drug-induced rhinitis, which does not usually resolve after delivery.21 However, there is insufficient evidence to establish an association between gestational rhinitis and an unfavorable outcome in pregnancy.7
Some authors, when comparing birth weight between atopic and non-atopic pregnant women, suggested that atopy, by favoring a Th2 pattern of immune response, would lead to better pregnancy outcomes.21 However, these results may have a confounding factor, as atopy has a higher prevalence in higher socioeconomic groups, who tend to have better nutritional care.7,22 In a national study of 230 pregnant women, there was no association between repeated miscarriage and atopy.23
TreatmentRegarding treatment, most of the studies show a consensus about the importance of educational measures as the first choice and as an adjuvant measure for the management of gestational rhinitis, mainly because symptoms resolve spontaneously after delivery.7,24 When advised early in pregnancy, patients tend to resort less to topical decongestants and will have lower chances of developing drug-associated rhinitis.25
Physical exercise has a well-established effect on nasal obstruction improvement,26 on the pregnant woman's weight control, and on sleep pattern improvement.24 Raising the head of the bed to 30°–45° may also help in improving nasal obstruction during the night. Additionally, nasal irrigation with saline solution provides good temporary symptom relief, although there are no specific studies for gestational rhinitis.7Table 2 summarizes non-pharmacological measures for the control of gestational rhinitis or rhinitis during pregnancy.
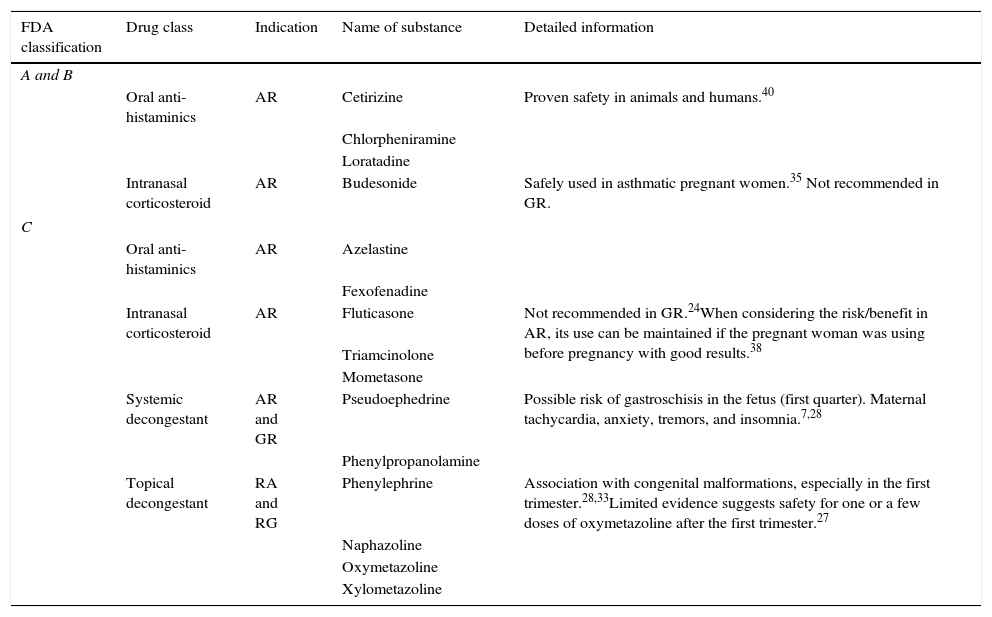
In relation to topical decongestants, they can be divided into short acting – phenylephrine; intermediate-acting – naphazoline; and long-acting drugs – oxymetazoline and xylometazoline. All these drugs are given a “C” classification by the Food and Drug Administration (FDA)27 – the complete FDA classification of drugs for use during pregnancy, shown in Table 3.
FDA drug classification during pregnancy.27
| Risk | Evidence |
|---|---|
| Risk A | There is no evidence of risk in pregnant women. |
| Well-controlled studies have showed no problems in the first trimester of pregnancy and there is no evidence of problems in the second and third trimesters. | |
| Risk B | There are no adequate studies in pregnant women. |
| Animal experiments have detected no risks. | |
| Risk C | There are no adequate studies in pregnant women. |
| There were some side effects on the fetus in animal experiments, but the product benefit may justify the potential risk during pregnancy. | |
| Risk D | There is evidence of risk in human fetuses. |
| Only to be used if the benefit outweighs the potential risk: life-threatening situations, or in cases of severe diseases for which safer drugs cannot be used, or if these drugs are ineffective. | |
| Risk X | Studies have disclosed abnormalities in the fetus or evidence of risk to the fetus. |
| The risks during pregnancy outweigh the potential benefits. Should not be used during pregnancy under any circumstances. | |
FDA, United States Food and Drug Administration.
A case–control study showed an association between the use of phenylephrine during pregnancy and the occurrence of congenital defects.28 Other studies, however, have failed to replicate this result.29–31 These studies have also failed to show adverse effects of intermediate and long-acting topical decongestants.
Thus, limited evidence suggests that oxymetazoline may be used occasionally in one or a few episodes of more severe nasal obstruction, which may be interfering with the patient's sleep, always at the lowest possible dose and preferably after the first trimester, and never close to delivery.28 In this sense, it has been shown that after a single dose of topical nasal oxymetazoline, there were no significant changes in either maternal blood pressure or heart rate, or changes in the uterine artery flow or umbilical vessels.32
However, in a more recent study, with 12,734 cases and 7606 controls conducted with children from the United States and Canada, an association was suggested between the use of topical nasal decongestants in the first trimester and hypertrophic pyloric stenosis, as well as the use of topical oxymetazoline in the second trimester with renal collecting system abnormalities in newborns.33 However, the study did not mention the amount and time of use of drugs.
Many authors recommend the use for no more than five, or even three days of topical nasal decongestants due to the proven risk of drug-induced rhinitis, which does not resolve after delivery.7 It is noteworthy that even the nighttime use only of decongestants can lead to this condition.24
The most commonly used systemic decongestant, pseudoephedrine, has also received a “C” classification by the FDA.27,28 Case–control studies have shown a statistically significant association between the use of pseudoephedrine or phenylpropanolamine during pregnancy and the risk of gastroschisis in newborns.34 These findings have not been replicated in later reviews.28 A recent case–control study, however, found a statistically significant association between the use of phenylephrine in the first trimester and endocardial wall closure defects, as well as between the use of phenylpropanolamine in the first trimester and external ear malformations and hypertrophic pyloric stenosis.33
Recommendations for the use of systemic decongestants during pregnancy vary between different countries. Given the lack of evidence to date and the side effects of systemic decongestants, which include tachycardia, anxiety, tremors, and insomnia in pregnant women, they should be especially avoided in the first trimester and their use should be considered as a risk/benefit ratio during pregnancy.7,28
As for topical corticosteroids, their use is well documented in other types of rhinitis (allergic or non-allergic) during pregnancy, but their effect on gestational rhinitis specifically has not been demonstrated and, therefore, is not recommended.7,24 Fluticasone propionate showed no significant effect in a double-blind placebo-controlled trial of 53 women with gestational rhinitis treated for eight weeks, when assessed by clinical scores of nasal complaints, peak expiratory flow, and acoustic rhinometry before and after treatment.35 The safety of inhaled budesonide, demonstrated by several studies in asthmatic pregnant women, has strongly suggested that the intranasal route of administration was also safe, attributing to that product category “B” of the FDA classification for use during pregnancy.36,37 However, given the fact that none of the topical corticosteroids appear to have adverse systemic effects at their therapeutic doses,36 the American College of Allergy, Asthma, and Immunology (ACAAI) even states that it is perfectly plausible to continue with a different topical corticosteroid, rather than budesonide, in a patient with allergic rhinitis who used the drug with good results before pregnancy.38
In relation to systemic corticosteroids, there are not enough studies to generate recommendations on the different types of rhinitis during pregnancy.7 Their use is considered an exception in these cases, for short periods, to alleviate decongestant use. Their use for longer periods of time or at higher doses can lead to adrenal insufficiency, low birth weight, and congenital malformations, especially cleft palate.39
Antihistamines, in turn, are reserved for cases of allergic or non-allergic eosinophilic rhinitis. In general, there are not enough data in the literature to suggest that antihistamines as a group have a deleterious effect on pregnancy.28 Chlorpheniramine, loratadine, and cetirizine have been recommended, as there have been studies in animals and humans demonstrating their safety.40
Sodium cromoglycate and topical ipratropium bromide, the latter used when there are severe complaints of rhinorrhea, have shown no teratogenic effects and can be safely used for allergic rhinitis during pregnancy.7 The use of anti-leukotrienes inhibitors (such as montelukast) for the treatment of allergic rhinitis during pregnancy is not recommended, as there are safer alternative drugs.28 Drug measurements in both gestational rhinitis and rhinitis during pregnancy are summarized in Table 4.
Safety level of the most commonly used drugs for the treatment of pregnant women with rhinitis.
| FDA classification | Drug class | Indication | Name of substance | Detailed information |
|---|---|---|---|---|
| A and B | ||||
| Oral anti-histaminics | AR | Cetirizine | Proven safety in animals and humans.40 | |
| Chlorpheniramine | ||||
| Loratadine | ||||
| Intranasal corticosteroid | AR | Budesonide | Safely used in asthmatic pregnant women.35 Not recommended in GR. | |
| C | ||||
| Oral anti-histaminics | AR | Azelastine | ||
| Fexofenadine | ||||
| Intranasal corticosteroid | AR | Fluticasone | Not recommended in GR.24When considering the risk/benefit in AR, its use can be maintained if the pregnant woman was using before pregnancy with good results.38 | |
| Triamcinolone | ||||
| Mometasone | ||||
| Systemic decongestant | AR and GR | Pseudoephedrine | Possible risk of gastroschisis in the fetus (first quarter). Maternal tachycardia, anxiety, tremors, and insomnia.7,28 | |
| Phenylpropanolamine | ||||
| Topical decongestant | RA and RG | Phenylephrine | Association with congenital malformations, especially in the first trimester.28,33Limited evidence suggests safety for one or a few doses of oxymetazoline after the first trimester.27 | |
| Naphazoline | ||||
| Oxymetazoline | ||||
| Xylometazoline | ||||
FDA, United States Food and Drug Administration; AR, allergic rhinitis; GR, gestational rhinitis.
Finally, surgery – volume reduction of inferior turbinates – has a limited role in rhinitis during pregnancy, reserved for the most severe cases, which include pregnant women with OSAS secondary to gestational rhinitis and failure of continuous positive airway pressure (CPAP) or other therapeutic methods.7
ConclusionBoth gestational rhinitis and the “rhinitis during pregnancy” are relatively common conditions that have gained importance in recent years, not only due to the discovery of association with maternal OSAS and possible unfavorable outcomes to the fetus, but also due to the important impact on the pregnant woman's quality life.7,41 Both the otorhinolaryngologist and the obstetrician should be alert for an early diagnosis and adequate treatment, considering the safety profile and current evidence of available measures and medications.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Caparroz FA, Gregorio LL, Bongiovanni G, Izu SC, Kosugi EM. Rhinitis and pregnancy: literature review. Braz J Otorhinolaryngol. 2016;82:105–11.