To manage the complications of irradiation of head and neck tissue is a challenging issue for the otolaryngologist. Definitive treatment of these complications is still controversial. Recently, hyperbaric oxygen therapy is promising option for these complications.
ObjectiveIn this study, we used biochemical and histopathological methods to investigate the efficacy of hyperbaric oxygen against the inflammatory effects of radiotherapy in blood and laryngeal tissues when radiotherapy and hyperbaric oxygen are administered on the same day.
MethodsThirty-two Wistar Albino rats were divided into four groups. The control group was given no treatment, the hyperbaric oxygen group was given only hyperbaric oxygen therapy, the radiotherapy group was given only radiotherapy, and the radiotherapy plus hyperbaric oxygen group was given both treatments on the same day.
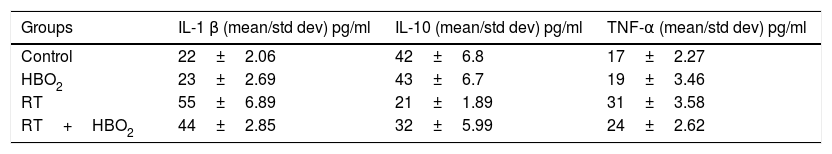
ResultsHistopathological and biochemical evaluations of specimens were performed. Serum tumor necrosis factor-α, interleukin-1β, and tissue inflammation levels were significantly higher in the radiotherapy group than in the radiotherapy plus hyperbaric oxygen group, whereas interleukin-10 was higher in the radiotherapy plus hyperbaric oxygen group.
ConclusionWhen radiotherapy and hyperbaric oxygen are administered on the same day, inflammatory cytokines and tissue inflammation can be reduced in an early period of radiation injury.
O manejo das complicações da irradiação do tecido da cabeça e pescoço é uma questão desafiadora para o otorrinolaringologista. O tratamento definitivo dessas complicações ainda é controverso. Recentemente, a oxigenoterapia hiperbárica tem sido uma opção promissora para estas complicações.
ObjetivoNesse estudo foram utilizados métodos bioquímicos e histopatológicos para investigar a eficácia do oxigênio hiperbárico contra os efeitos inflamatórios da radioterapia no sangue e nos tecidos laríngeos, quando a radioterapia e oxigênio hiperbárico são administrados no mesmo dia.
MétodosTrinta e dois ratos Wistar albinos foram divididos em quatro grupos. O grupo controle não recebeu tratamento, o grupo de oxigênio hiperbárico recebeu apenas oxigenoterapia hiperbárica, o grupo de radioterapia recebeu apenas radioterapia e o grupo de radioterapia com oxigênio hiperbárico recebeu ambos os tratamentos no mesmo dia.
ResultadosForam realizadas avaliações histopatológicas e bioquímicas dos espécimes. Os níveis séricos de fator de necrose tumoral-α, interleucina-1β e inflamação tecidual foram significativamente maiores no grupo de radioterapia do que no grupo de radioterapia mais oxigênio hiperbárico, enquanto que a interleucina-10 foi maior no grupo de radioterapia mais oxigênio hiperbárico.
ConclusãoQuando a radioterapia e o oxigênio hiperbárico são administrados no mesmo dia, as citocinas inflamatórias e a inflamação tecidual podem ser reduzidas no período inicial da radiação.
Head and neck cancers are the most frequently occurring cancers worldwide. In epidemiologic studies, they are ranked 10th globally, and their incidence has increased considerably in the past 10 years. The management of head and neck cancer is complex and requires a multidisciplinary approach.1 Radiotherapy is used as a primary or adjuvant treatment option for the treatment of head and neck cancers. Irradiation of the neck region can be performed in the presence of neck lymph node metastasis from the larynx, or cancers of any other region, or in the various stages of laryngeal cancer.2 Apart from the therapeutic nature of radiotherapy, it also has definite hazardous effects on the surrounding tissues and these effects can occur at either early or late stages. Dry mouth, mucositis, Soft Tissue Radionecrosis (STRN), Osteoradionecrosis (ORN), and Laryngeal Radionecrosis (LRN) are some of the examples of these side effects.2,3
Radiation causes the release of inflammatory mediators, marking the beginning of the inflammatory process, and these result in an increase in oxidizing activity in the damaged tissues.4 Radiation not only causes damage to tissues through Reactive Oxygen Species (ROS) and ionization, but also through the caspase pathway and cytochrome activation, and as a result, necrosis develops in the damaged tissues from ischemia and apoptosis.5,6 The cell tries to combat this damaging effect by activating its defense system (anti-inflammatory cytokines, antioxidant activity).7
Recently, a number of scientific studies on the management of side effects of radiotherapy have been conducted, and antioxidant herbal medicine, various chemical agents, vitamins, exogenous antioxidant molecules, and Hyperbaric Oxygen (HBO2) have been used in these studies.6,8–11 HBO2 has been widely used for the treatment of various diseases for decades. Treatment of diabetic wounds, flap necrosis, and sudden hearing loss are some of the prominent examples.12–14
In this study, we used biochemical and histopathologic methods to investigate the efficacy of hyperbaric oxygen against the early inflammatory effects of radiotherapy on the laryngeal tissue when radiotherapy and HBO2 are administered on the same day.
MethodsThis study was reviewed and approved by the Institutional Animal Care and Use Committee and Local Instuitional Review Board. The number of rats in each group was restricted by the ethics committee.
Experimental designThirty-two Wistar Albino rats were divided into four groups. The control group (n=8) was given no treatment during the study period. The HBO2 treatment group (HBO2) (n=8) was given only HBO2 throughout the study. The Radiotherapy (RT) group (n=8) was given only RT throughout the study. The RT plus HBO2 group (RT+HBO2) (n=8) was given both RT and HBO2 on the same day.
AnimalsWistar albino rats (female, 230±20g) were housed under standard conditions (20±1°C room temperature, 60±10% humidity, and a 12/12h light/dark cycle) in regular cages and allowed free access to food and water. All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the US Public Health Service.
Neck area irradiationRadiotherapy was applied under general anesthesia with 10mg/kg ketamine and 8mg/kg xylazine intraperitoneally (i.p.) to immobilize the rats prior to radiation exposure. The animals were placed on a plexiglass tray and stabilized in the supine position. The neck region of each rat was defined by simulation and irradiated with 2 Gray (Gy) per minute, for a total dose of 18Gy with 6MV photon beams (linear accelerator, Siemens, Primus). The source-axis distant technique was used, and the distance from the source center to the larynx was 100cm. A 10mm bolus was applied above the neck to compensate for dose depth. Each rat was exposed to a single dose of radiation. The animals were returned to their home cages following irradiation.
HBO2 treatmentThe animals were treated with HBO2 in the research animals’ pressure room in the Animal Care and Research Unit. The rats were given HBO2 once a day, 6 days a week, for a period of 4weeks. The rats were taken in their cages to the pressure room, and, after a 10min 100% oxygen ventilation, the cabin pressure was regulated at 2.4 absolute atmosphere for 10min. Ventilation was provided at intervals throughout the 90min treatment. Exits from the pressure room took 10min. The rats in the RT+HBO2 group were started on HBO2 treatment before radiotherapy on the same day that RT was given.
Euthanasia protocolFour weeks later, after the administration of RT, all the rats were placed under general anesthesia using 10mg/kg ketamine and 8mg/kg xylazine i.p. The rats’ hearts were punctured and blood withdrawn. Thereafter, they were immediately decapitated and their laryngeal tissues removed surgically.
Histopathological and immunohistochemical evaluationTwenty-four hours of tissue fixation in 10% formaldehyde was performed, and then, the tissues were embedded in paraffin blocks. The processed tissues were sliced in an microtome equipment. Five-micron-thick cross-sections from all of the blocks were printed onto lysine-coated slides. One cross-section from each block was taken for staining with hematoxylin and eosin (H+E); one cross-section was stained with immunohistochemical staining to show Leukocyte Common Antigen (LCA). Thermo AntiLCA Polyclonal Kit (PA5-23528, USA) was used to show LCA. All the preparations were analyzed with an Olympus BX51 light microscope by the same pathologist blinded to these groups. A general cartilage evaluation was performed using H+E staining, and the level of inflammation was evaluated using immunohistochemical LCA staining.
Inflammation and metaplasia levels were calculated as follows: 0–3. 0 – no inflammation, 1 – low inflammation, 2 – moderate inflammation, 3 – severe inflammation, 0 – no metaplasia, 1 – there is metaplasia.
Biochemical evaluationMeasurement of proinflammatory and anti-inflammatory cytokines and oxidant values in rat plasma was conducted. The blood samples were transferred to K3-EDTA tubes and centrifuged at 1000×g for 10min at +4°C. Plasma samples were separated and stored at −0°C. The plasma samples were later defrosted for use. TNF α (Biolegend, San Diego, CA, USA), IL1-β and IL-10 (Boster Biological Technology, CA, USA) levels were measured in the homogenized samples by enzyme-linked immunosorbent assay (ELISA) using industrial kits as instructed by the manufacturers.
Statistical analysisData were analyzed using the SPSS 23.0 (USA) packet program. For comparison within the groups, Kruskal–Wallis variance analysis was used. To determine significant differences between the groups, the Mann–Whitney U test with Bonferroni's correction was used. p<0.05 was accepted as statistically significant.
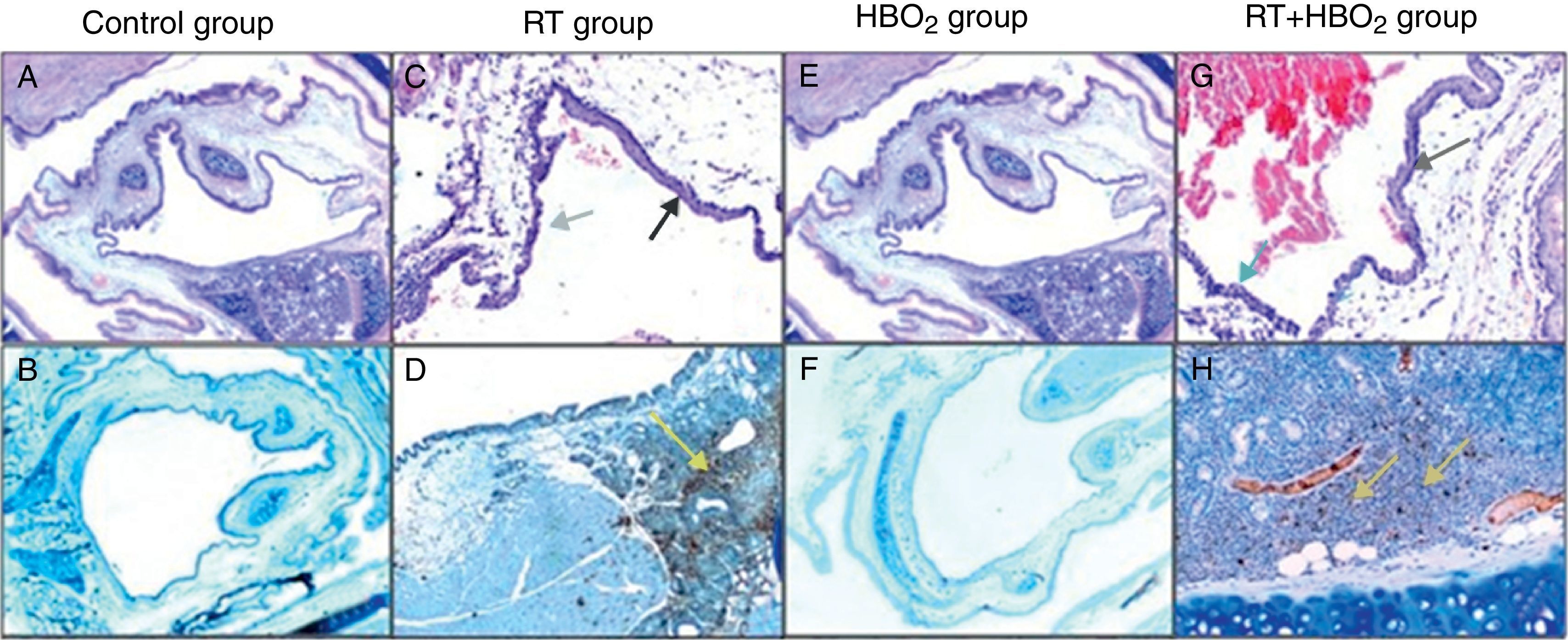
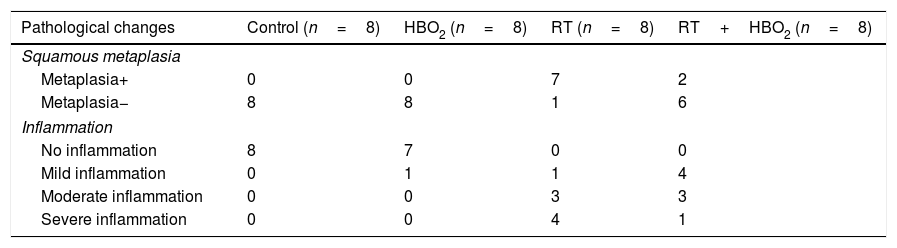
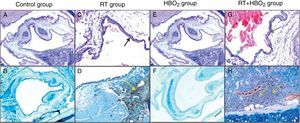
ResultsHistopathological and immunohistochemical findingPathological variations and their distributions in the specimens in all the groups are shown in Table 1 and Fig. 1. Inflammation and metaplasia seen in laryngeal tissue were not statistically significant between the control and HBO2 groups (p>0.05). The rate of inflammation and metaplasia in the cartilage tissue were significantly higher in the RT group compared to the control, HBO2, and RT+HBO2 groups (p<0.05). The cartilage tissue of the rats in the RT+HBO2 group had a significantly higher inflammation and squamous metaplasia level than the control and HBO2 groups.
Squamous metaplasia and inflammation levels of the rat groups.
| Pathological changes | Control (n=8) | HBO2 (n=8) | RT (n=8) | RT+HBO2 (n=8) |
|---|---|---|---|---|
| Squamous metaplasia | ||||
| Metaplasia+ | 0 | 0 | 7 | 2 |
| Metaplasia− | 8 | 8 | 1 | 6 |
| Inflammation | ||||
| No inflammation | 8 | 7 | 0 | 0 |
| Mild inflammation | 0 | 1 | 1 | 4 |
| Moderate inflammation | 0 | 0 | 3 | 3 |
| Severe inflammation | 0 | 0 | 4 | 1 |
Representative photomicrographs of cartilage histology in the control, HBO2, RT and RT+HBO2 groups. Control group: (A) general appearance of H&E (×20); (B) Immunohistochemical LCA (×20). RT group: (C) H&E (×100) black arrow squamous metaplasia – blue arrow columnar epithelium. (D) Immunohistochemical LCA (×40) yellow arrow LCA positive lymphocytes. HBO2 group: (E) H&E (×20) general appearance; (F) Immunohistochemical LCA (×20). RT+HBO2 group: (G) H&E (×100) black arrows quamous metaplasia – blue arrow columnar epithelium; (H) Immunohistochemical LCA (×100) yellow arrow LCA positive lymphocytes.
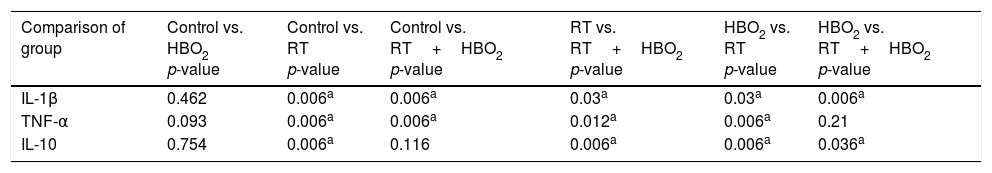
Biochemical parameter results of all groups are shown in Table 2. There were no statistically significant differences in TNF-α, IL1-β, and IL-10 values between the control and HBO2 groups (p>0.05). TNF-α and IL1-β values were significantly higher in the RT group compared to the control group (p<0.05), whereas IL 10 was higher in the control group (p<0.05). TNF α and IL1-β values were significantly higher in the RT group than in the RT+HBO2 group (p<0.05), whereas IL-10 was higher in the RT+HBO2 group (p<0.05). IL1-β values were significantly higher in the RT+HBO2 group than in the HBO2 group (p<0.05), whereas IL-10 was higher in the HBO2 group (p<0.05). There was no significant difference in TNF-α values between these two groups (p>0.05). TNF-α and IL1-β were significantly higher in the RT+HBO2 group than in the control group (p<0.05). There was no statistically significant difference in IL-10 between these two groups (p>0.05) (Tables 2 and 3).
p-Values showing comparisons between the important groups.
Signs and symptoms after the completion of RT treatment were described as being early if they occurred within the first 3 months and late if they occurred after the first 6 months. The early and late effects of RT on the normal tissue are different with regard to both pathophysiology and clinical presentation. Changes related to the early effects of RT are either a result of increasing cell death from DNA injury or formation of ROS, whereas late changes related to RT are a result of vascular injury and fibrosis. These changes are usually progressive.15–17 In head and neck cancers, radiotherapy is administered in lower doses and in repeated sessions. The aim of administering a single high dose of radiotherapy in our study was to successfully induce radiation injury and the radiation dose that we administered was decided by considering previous similar studies.7 In our study, HBO2 treatment was started on the same day as radiotherapy because HBO2 was given for protective effect, not for the treatment of damaged tissue.
HBO2 is usually used to treat the late period radiation effects of radiotherapy. Narozny et al.18 reported that of 548 patients who were given RT to treat head and neck cancer, grade 3 and 4 chondroradionecrosis developed in six patients, and all had symptom remission after conventional treatment followed by HBO2. In another retrospective review, it was reported that none of five chondroradionecrosis patients treated with HBO2 required laryngectomy, and two of the four patients from this group who were dependent on tracheotomy were decanulated.16 Filntisis et al.17 administered adjuvant HBO2 treatment to 18 patients with grade 3 and 4 radionecrosis, and 13 (72.2%) of these patients showed major recovery after HBO2 treatment; 5 (27.8%) patients required a total laryngectomy due to unsuccessful treatment. Filntisis reported that HBO2 treatment is a safe and easy way to use adjuvant treatment in larynx radionecrosis that is resistant to conservative methods. Furthermore, in a study by Roh et al.,19 of six patients diagnosed with chondroradionecrosis and treated with early debridement and HBO2, recovery was seen in five patients, whereas one patient had to undergo a total laryngectomy due to failed treatment. In a study by Niezgoda et al.20 HBO2 was used in the treatment of various tissue injuries caused by radiation, and recovery was obtained in laryngoradionecrosis combined with a number of other tissue injuries.
In all the above-mentioned studies, HBO2 was given as treatment after injury had occurred, which mostly involved late radiation injury. In our study, HBO2 was given at the same time as radiotherapy to determine its effects on tissue inflammation the protection of tissue from inflammation. Consequently, it considerably reduced inflammation. When the rats were decapitated on the fourth week, inflammation that occurred due to radiotherapy was an early effect, and to some point, HBO2 had suppressed inflammation that could cause tissue injury. Six months must pass for the late effects to be realized.
Studies have researched the anti-inflammatory effect of HBO2. In a study by Chen et al.,21 the middle cerebellar artery of rats was clamped, rendering the brain hypoxic. HBO2 was given for treatment and was shown to decrease inflammatory cytokines and increase antioxidant activity. In our study, HBO2 decreased inflammatory cytokines and tissue inflammation but increased anti-inflammatory cytokine. In another study, colon injury was induced in rats by administering dextran sulfate sodium, and then HBO2 was given.22 HBO2 reduced pro inflammatory cytokines and suppressed inflammation. Arslan et al.23 induced acoustic trauma in the ear, treated it with dexamethasone and HBO2, and then compared these two agents in their study. They reported that when HBO2 is given, especially in the first 24h, it can be as effective as dexamethasone as an anti-inflammatory agent. The anti-inflammatory effect of HBO2 is a common factor in these studies, and their results are similar to our present results.
Our study has some limitations. First, the early period anti-inflammatory effect of HBO2 against radiation injury was evaluated, but this anti-inflammatory effect on the late effects of radiation, such as soft tissue necrosis was not evaluated. Furthermore, the anti-inflammatory effect of HBO2 on the antitumor effects of radiotherapy is not known. The other limitation is that the effects of low and multiple radiation doses could not be predicted because a single large dose was given in this study.
ConclusionHBO2 plays an anti-inflammatory role in the early period after radiotherapy injury in tissue when given on the same day as radiotherapy. Further studies are needed to show the late effects when both agents are given on the same day as well as the effects on the tumoral tissue.
Ethical considerationsThe study has been conducted in the local Experimental Animals Research and Application Center of the Faculty of Medicine. The approval of the ethical board has been taken (number of approval of the ethics committee: 2015-008).
Conflicts of interestThe authors declare no conflicts of interest.
This research received no specific grant from any funding agency, commercial or not-for profit sectors. The English of this document was edited by Elsevier English Editing.
Please cite this article as: Arıcıgil M, Dündar MA, Yücel A, Arbağ H, Arslan A, Aktan M, et al. Anti-inflammatory effects of hyperbaric oxygen on irradiated laryngeal tissues. Braz J Otorhinolaryngol. 2018;84:206–11.
Peer Review under the responsibility of Associação Brasileira de Otorrinolaringologia e Cirurgia Cérvico-Facial.