The use of handheld devices that assess peripheral arterial tonometry has emerged as an auxiliary method for assessment and diagnosis of obstructive sleep apnea syndrome.
ObjectiveTo evaluate the accuracy of peripheral arterial tonometry in the diagnosis of obstructive sleep apnea.
MethodsContemporary cohort cross-sectional study. Thirty patients with suspected obstructive sleep apnea underwent peripheral arterial tonometry and assisted nocturnal polysomnography concomitantly.
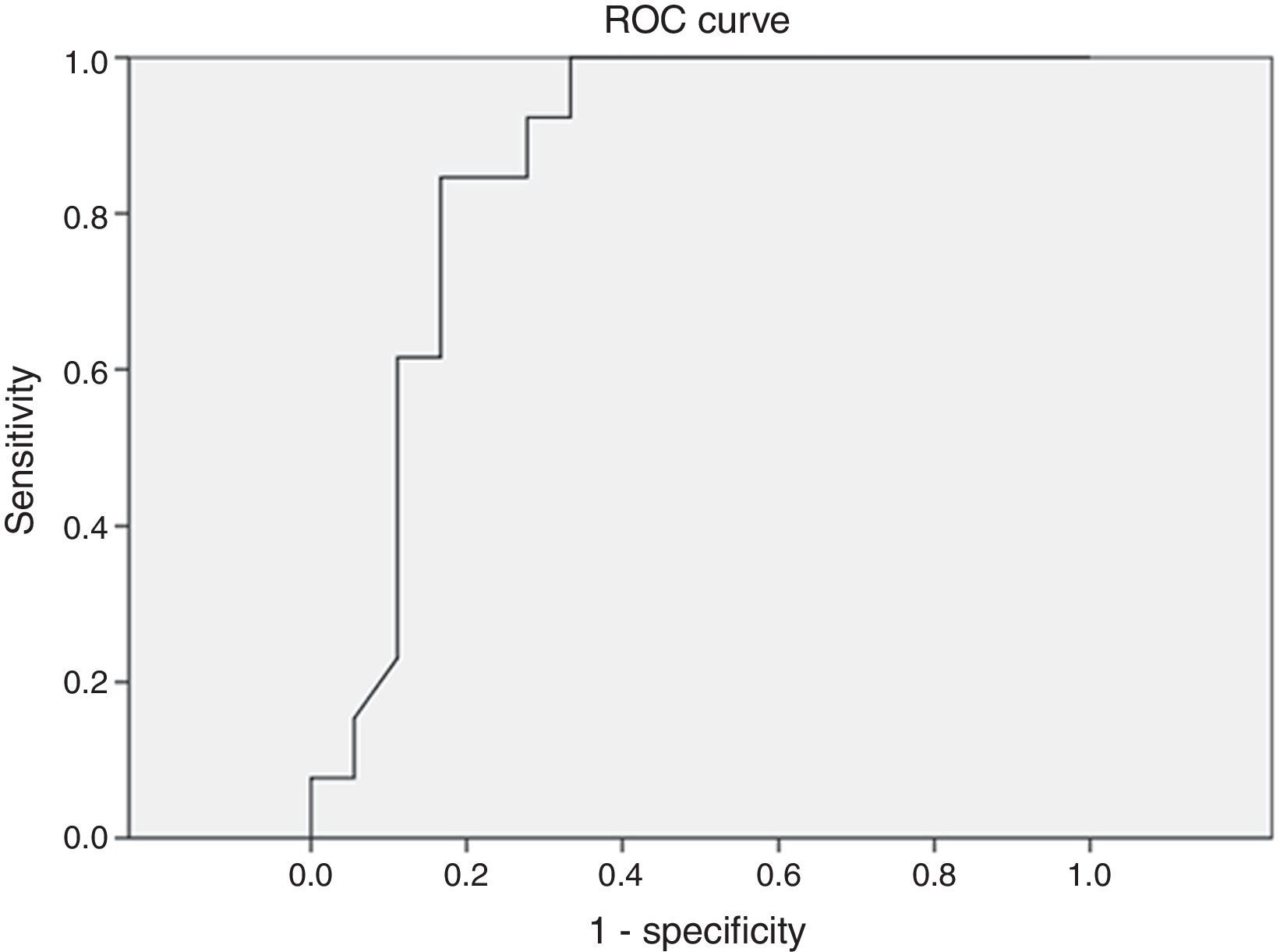
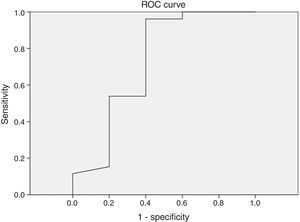
ResultsThe mean apnea/hypopnea index by peripheral arterial tonometry was significantly higher than that by polysomnography (p<0.001), but the values of both sleep studies were significantly correlated (r=0.762). There was a high correlation between variables: minimum oxygen saturation (r=0.842, p<0.001), oxygen saturation<90% (r=0.799, p<0.001), and mean heart rate (r=0.951, p<0.001). Sensitivity and specificity were 60% and 96.2% (AUC: 0.727; p=0.113), respectively, when at a threshold value of 5 events/h. In severe cases (≥30 events/h), the result was a sensitivity of 77.8% and a specificity of 86.4% (AUC: 0.846, p=0.003).
ConclusionPeripheral arterial tonometry is a useful portable device for the diagnosis of obstructive sleep apnea; its accuracy is higher in moderate and severe cases.
A utilização de dispositivos portáteis, que avaliam a tonometria arterial periférica, surge como método adjuvante para avaliação e diagnóstico da síndrome da apneia obstrutiva do sono.
ObjetivoAvaliar a acurácia da tonometria arterial periférica no diagnóstico da apneia obstrutiva do sono.
MétodoEstudo de coorte contemporânea com corte transversal. Trinta pacientes com suspeita de apneia obstrutiva do sono foram submetidos a tonometria arterial periférica e a polissonografia noturna assistida simultaneamente.
ResultadosA média do índice de apneia/hipopneia pela tonometria arterial periférica foi significativamente maior do que a da polissonografia (p<0,001), porém os valores de ambos os estudos do sono foram significativamente correlacionados (r=0,762). Houve alta correlação entre as variáveis: saturação mínima de oxigênio (r=0,842, p<0,001), saturação de oxigênio<90% (r=0,799, p<0,001) e média de frequência cardíaca (r=0,951, p<0,001). A sensibilidade e especificidade foram 96,2% e 60% (AUC: 0,727, p=0,113), respectivamente, quando limiar de 5 eventos/hora. Nos casos graves (≥ 30 eventos/hora), o resultado foi uma sensibilidade de 77,8% e uma especificidade de 86,4% (AUC: 0,846, p=0,003).
Conclusão: A tonometria arterial periférica é um dispositivo portátil útil no diagnóstico da apneia obstrutiva do sono e sua acurácia é maior nos casos moderados e graves.
Obstructive sleep apnea syndrome (OSAS) is a form of sleep-disordered breathing (SDB) characterized by recurrent episodes of partial or complete obstruction of the upper airways during sleep, which lead to intermittent hypoxemia, transient hypercapnia, and frequent awakenings associated with clinical signs and/or symptoms.1 It is estimated that the prevalence of OSAS in the adult population is 2–4%.2 A recent epidemiological study showed a high prevalence of OSAS in the adult population of São Paulo (32.8%).3
The most common signs and symptoms of OSAS are snoring, excessive daytime sleepiness, and breathing pauses during sleep, in addition to cardiovascular disorders such as hypertension, ischemic heart disease, stroke, heart failure, arrhythmias, and sudden death.4 Early diagnosis is paramount. Loss of cognitive functions (such as concentration, attention, and memory), and of executive function are often observed, as well as mood swings, irritability, depression, and anxiety.5 Proper diagnosis and correct treatment of OSAS leads to significant improvement in symptoms, reducing the risks associated with this disease.6,7
Polysomnography (PSG) is considered the gold-standard for diagnosis of OSAS, which combines the overnight monitoring of sleep stages with a continuous register of airflow, chest and abdominal ventilatory movements, heart rate, oxygen saturation, snoring, muscle tone, and leg movement.3,8 The number of events (apneas or hypopneas) per sleep hour is known as the apnea–hypopnea index (AHI), which is widely used in the assessment of severity of the syndrome.9
There are several portable devices used for evaluation and diagnosis of OSAS. Some of these devices assess peripheral arterial tonometry (PAT), a physiological sign of changes in the autonomic nervous system that occur during sleep. In addition to PAT, these handheld monitors assess heart rate, oxygen saturation, and actigraphy. An automatic algorithm analyzes the amplitude of PAT signal that, in association with variations in heart rate and oxygen saturation, identify respiratory events. The result of this algorithm determines apnea/hypopnea index (AHI) and respiratory disturbance index (RDI), using specific signal patterns.10,11
Sleep studies with PSG are costly, because they require a full sleep laboratory and a specialized team. Therefore, alternative diagnostic methods are being developed, such as peripheral arterial tonometry, enabling its realization in patient's own home in order to reduce costs and maintain the same efficiency in the diagnosis of sleep disorders.3,10 The mean cost of these two diagnostic methods was significantly different (PSG, US$ 2252.73; SD, US$ 877.40; PAT, US$ 895.74, SD US$ 975.00; p<0.001).12
The aim of this study was to evaluate the accuracy of PAT in the diagnosis of obstructive sleep apnea.
MethodsThis was a historical, single-blind, cross-sectional cohort study conducted at a private hospital in São Paulo. Whole-night polysomnography was performed in a hospital environment concomitantly with peripheral arterial tonometry recordings with a Watch-PAT-200® device (Itamar Medical Ltd. – Caesarea, Israel) in patients with suspicion of OSAS (symptoms such as snoring, excessive daytime sleepiness, and apnea witnessed during sleep by the spouse). Thirty-three patients were subjected to monitoring from October to November of 2013. Of these, three patients were excluded for operational problems in which there was no technical interference. Patients were carefully pre-clinically evaluated to exclude those with central or mixed apnea, moderate-to-severe lung disease, neuromuscular disease, and congestive heart failure. Parameters of sleep architecture and respiratory measures were evaluated and compared through analysis by polysomnography and with the use of a Watch-PAT-200® device. Test interpretation was carried out by two physicians experienced in sleep medicine.
Polysomnography procedures were staged according to the rules established in the American Academy of Sleep Medicine (AASM) manual (2007); apnea is defined as an airflow reduction>90% for at least 90% of the episode, for a minimum of 10s, and measured by a thermal sensor.13 For hypopnea, an “alternative” rule was used, which consists in an airflow reduction>50%, accompanied by oxygen desaturation>3% and/or awakening,1 which is best observed by nasal cannula.
Watch-PAT® consists of a device placed on the wrist of the patient's non-dominant hand, connected to a probe applied on the ipsilateral index finger. The device has a snore and position sensor fixed with adhesive on the chest and sternal notch, as well as an oximeter placed on the ring finger also of the patient's non-dominant hand. The device's specific software (zzzPat®) reads the exam. The zzzPAT® algorithm is based on 14 features from two series of PAT amplitude and from inter-pulse periods (IPP). In association with actigraphy, these data allow staging of patient's sleep into the following stages: wakefulness, light sleep, deep sleep, and rapid eye movement (REM) sleep.
In statistical analysis, means and standard deviations for continuous variables from assisted nocturnal PSG and Watch-PAT® were described using Spearman correlation and Bland–Altman plots. The accuracy was analyzed using ROC curves. The statistical program used was SPSS v.18. The p-value was considered significant when<0.05. This study was approved by the Ethics in Research Committee of this institution, under No. 437912.
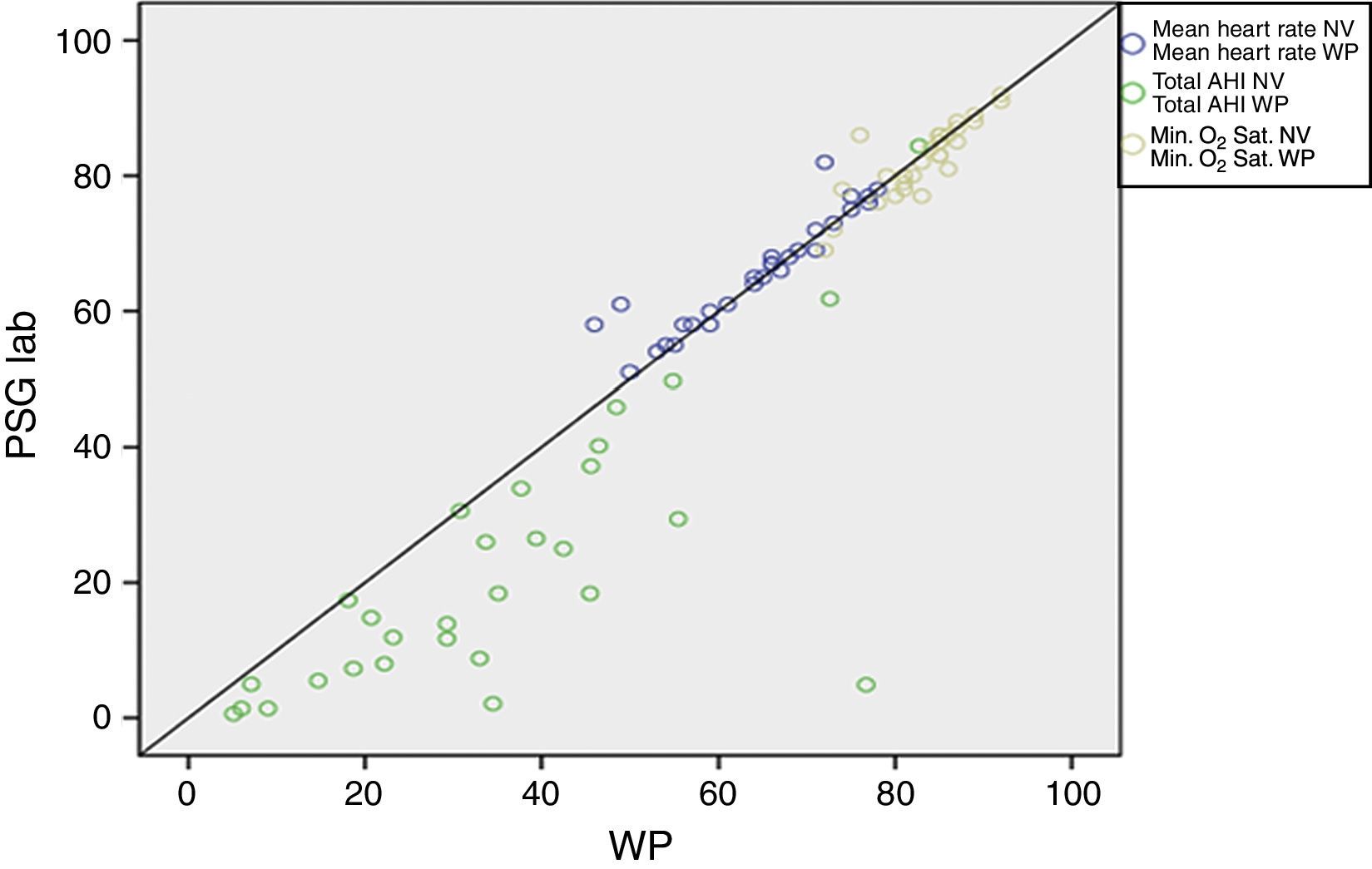
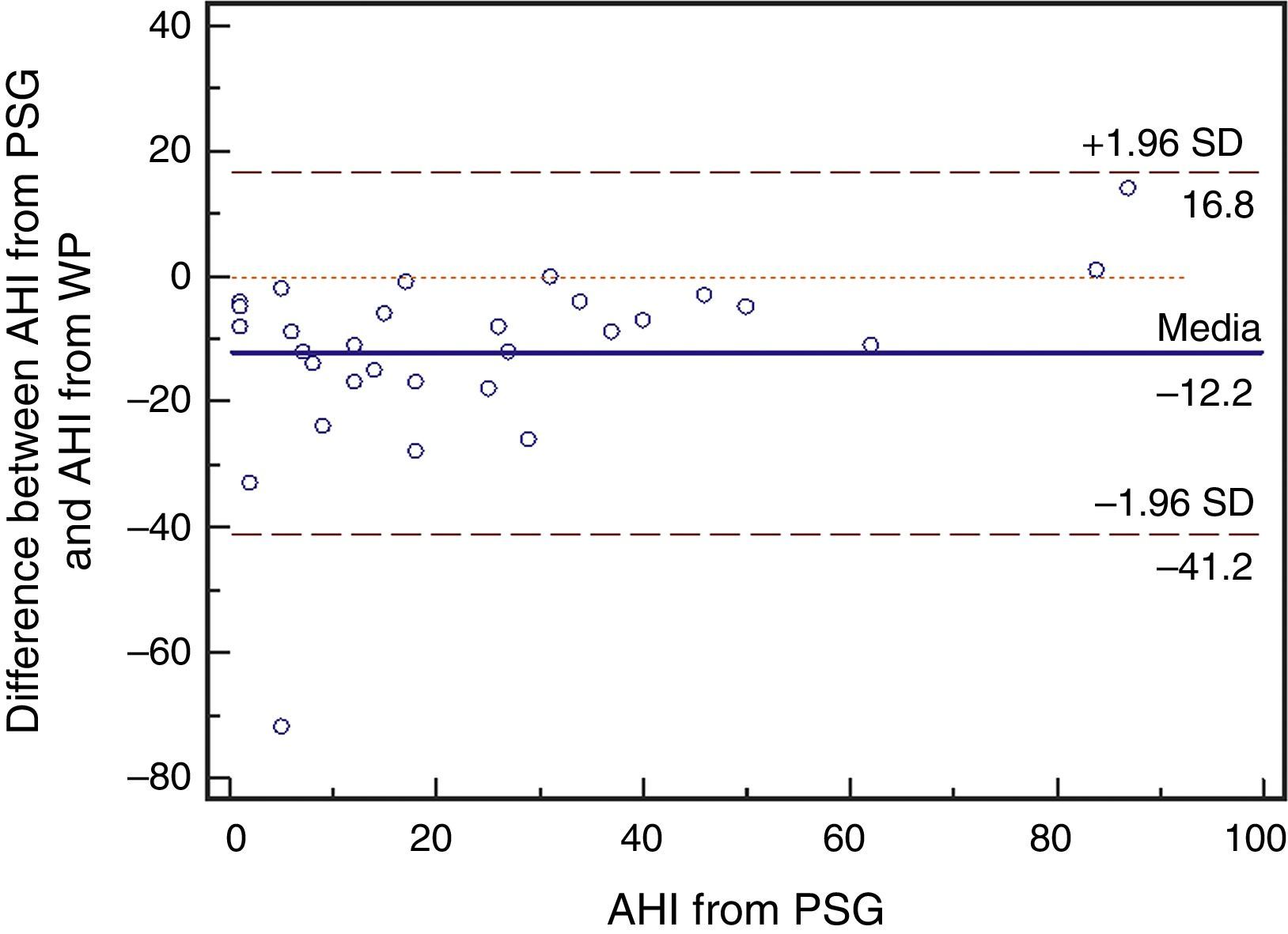
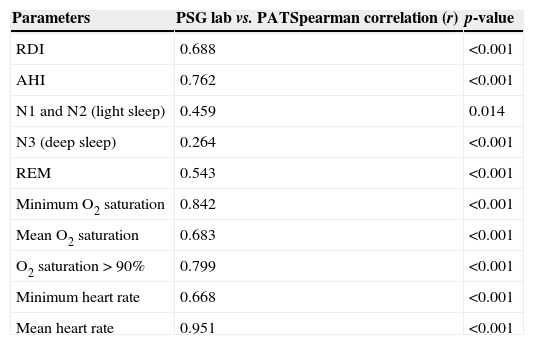
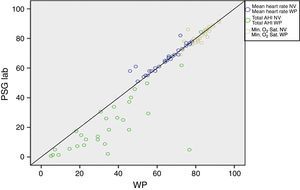
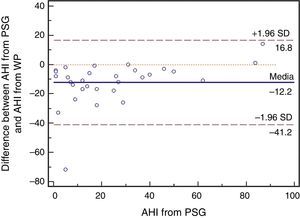
ResultsA total of 30 adult patients with suspected OSAS, 20 (66.7%) male and 10 (33.3%) female subjects with mean age of 42.8±12.32 years (range 24–71 years) were evaluated. A high correlation among values of the following variables from assisted nocturnal PSG and peripheral arterial tonometry was found: AHI (r=0.762, p<0.0001), minimum oxygen saturation (r=0.842, p<0.0001), oxygen saturation<90% (r=0.799, p<0.001), and mean heart rate (r=0.951, p<0.0001) (Table 1 and Fig. 1). With the use of Bland–Altman plots for AHI obtained from PAT (WP) vs. AHI from PSG, a good agreement between AHI values was observed (Fig. 2).
Spearman correlation among values of laboratory polysomnography (PSG lab) and peripheral arterial tonometry (PAT) parameters.
| Parameters | PSG lab vs. PATSpearman correlation (r) | p-value |
|---|---|---|
| RDI | 0.688 | <0.001 |
| AHI | 0.762 | <0.001 |
| N1 and N2 (light sleep) | 0.459 | 0.014 |
| N3 (deep sleep) | 0.264 | <0.001 |
| REM | 0.543 | <0.001 |
| Minimum O2 saturation | 0.842 | <0.001 |
| Mean O2 saturation | 0.683 | <0.001 |
| O2 saturation>90% | 0.799 | <0.001 |
| Minimum heart rate | 0.668 | <0.001 |
| Mean heart rate | 0.951 | <0.001 |
RDI, breathing disturbance index; AHI, apnea/hypopnea index; REM, rapid eye movement.
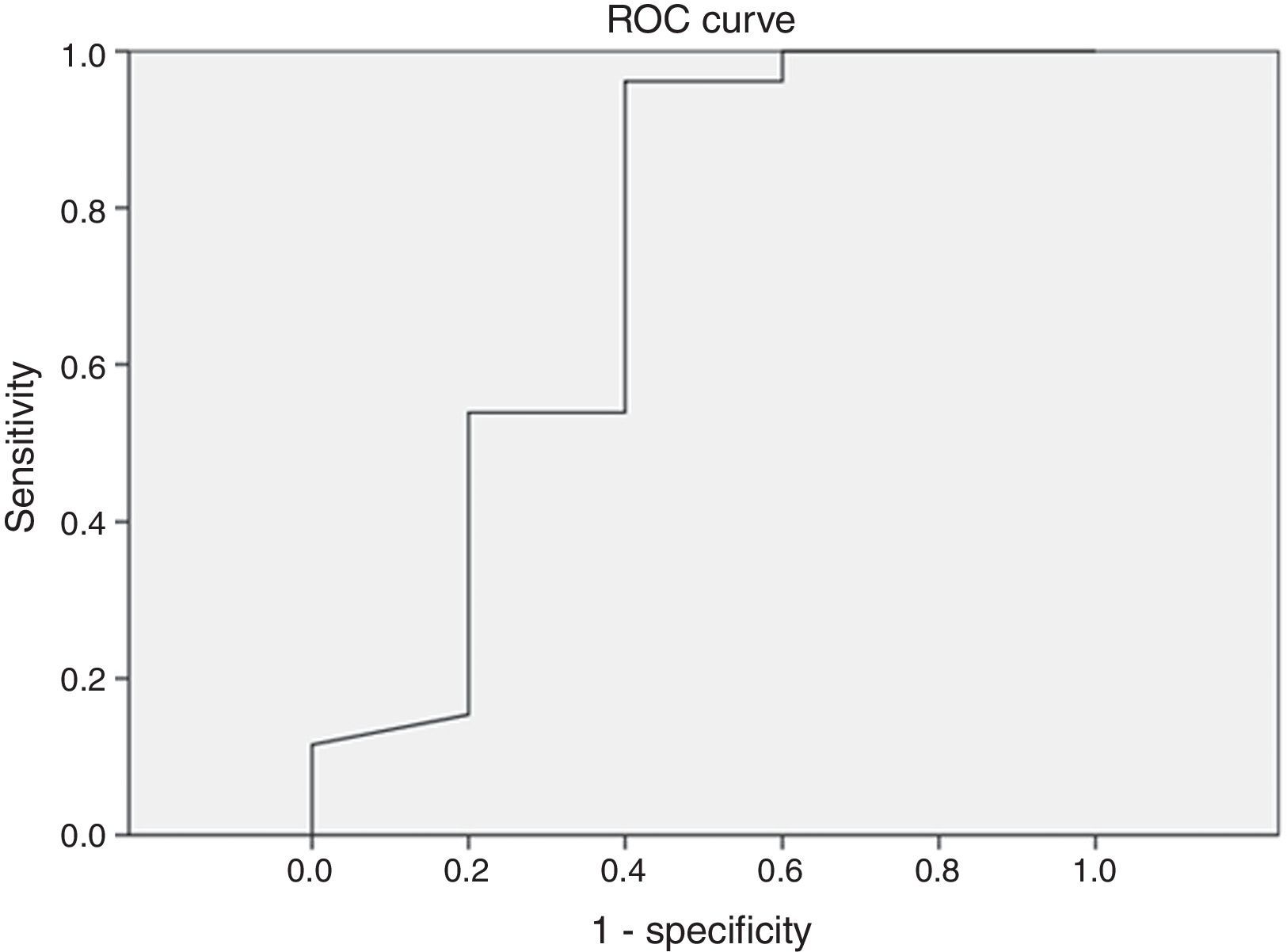
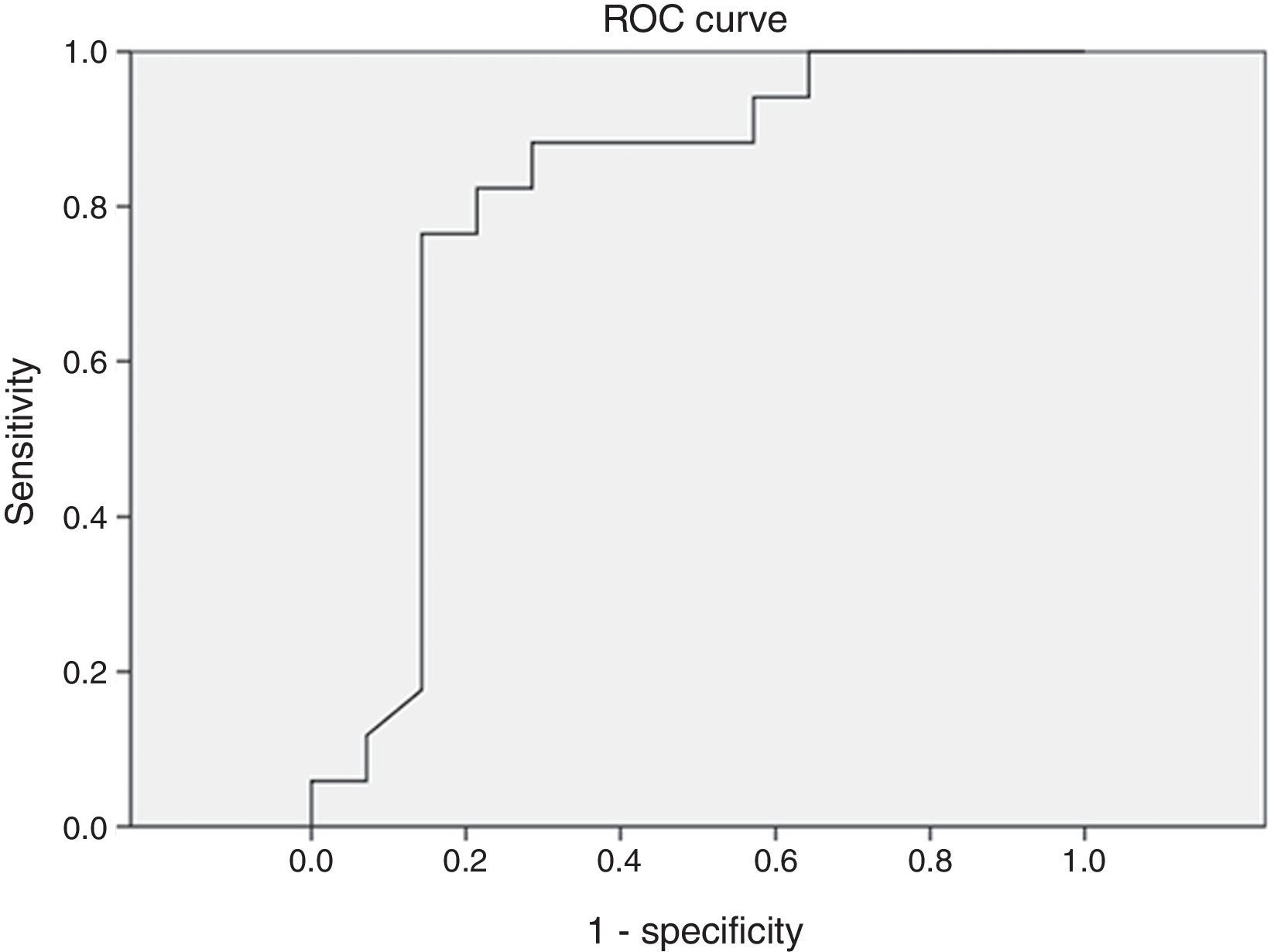
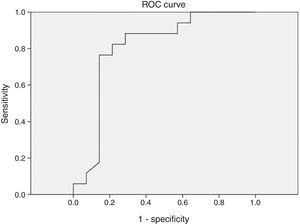
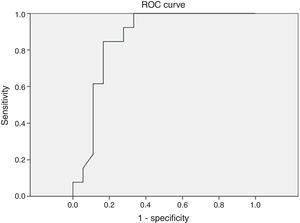
Different cutoff values for AHI were established in order to evaluate sensitivity and specificity rates of peripheral arterial tonometry tests. For AHI≥5 events/h, the sensitivity was 96.2% and the specificity, 60% (AUC 0.727, p=0.113) (Fig. 3). For AHI≥10 events/h, the sensitivity was 70% and the specificity 73.7% (AUC 0.761, p=0.018). For AHI≥15 events/h, the sensitivity was 82.4% and the specificity 78.6% (AUC 0.805, 0.004) (Fig. 4). For AHI≥20 events/h, the sensitivity was 84.6% and the specificity 83.3% (AUC 0.861, p=0.001) (Fig. 5). For AHI≥30 events/h, the sensitivity was 77.8% and the specificity 86.4% (AUC 0.846, p=0.003). It worth noting that accuracy of PAT is higher when the value of AHI is greater than 20 events/h compared to lower cutoff scores.
DiscussionThe method used to assess peripheral arterial tonometry distinguishes REM sleep from NREM sleep. In REM sleep, there is an attenuation of PAT signal amplitude in relation to NREM sleep.14 As in deep sleep, PAT signal variability is lower than in REM sleep; and superficial sleep has greater variability than in REM, and the recording may also distinguish between deep NREM sleep vs. superficial NREM sleep.15 Hedner et al. showed that PAT signal is useful to distinguish wakefulness from sleep, and for stratification in light sleep, deep sleep, and REM stages. There was statistical significance between N3 and REM sleep in this study.16 Sleep time is critical to determine true DRI. Arterial tonometry detects the state of sleep/wakefulness and REM sleep stage through the “total sleep time”; this provides an accurate estimate of sleep architecture. The detection of automatic sleep time by this diagnostic method is determined from total recording time – (waking time+invalid sign times). Several validation studies of PAT as a diagnostic method of OSAS have been published. Schnall et al. reported a high correlation between AHI values in laboratory polysomnography and PAT (r=0.92, p<0.0001).17 Several other authors also found high correlation between AHI values from PAT and laboratorial polysomnography.5,8–11,14–16 Pang et al. found a good correlation between AHI (r=0.9288, p<0.0001) and minimal oxygen saturation (r=0.9891, p<0.0001), and between PSG and the portable monitor that evaluates PAT18; O’Donnell et al. experimentally induced an obstruction of upper airways and concluded that the airflow obstruction characteristic of OSAS patients results in attenuation of the PAT signal.19
The major difference between this portable monitor and other existing devices is that the identification of respiratory events, such as hypopnea and apnea, is performed by means of changes in peripheral arterial tone signal, without the need for conventional measures of airflow and respiratory effort.8–11 However, peripheral arterial tonometry does not distinguish between apneas and hypopneas. Choi et al. found high correlation with the severity of AHI (Kendall tau – b=0.897, p<0.001) and PSG.5 While the severity of OSAS is defined as the sum of the two events in relation to total sleep time, this non-distinction is hardly relevant, since apneas and hypopneas lead to similar clinical consequences.
In the present study, good correlation was found between AHI values, minimum oxygen saturation, and mean heart rate, when comparing the parameters of laboratory polysomnography and peripheral arterial tonometry. A good agreement was found between AHI values of the two recordings. The sensitivity and specificity of AHI values obtained by PAT were higher when AHI was greater than 20 events/h. When comparing patients with mild and moderate to severe OSAS according to AHI, Ceylan et al. obtained sensitivity and specificity of 88.2 and 80.0%, respectively. There was a high correlation between PSG and PAT with respect to AHI, respiratory disturbance index, and oxygen desaturation index.20
The device for peripheral arterial tonometry evaluation tended to overestimate AHI values in mild cases of OSAS (Figs. 3 and 4). In the present study, there was a higher sensitivity to resaturation rate. Sometimes, the decrease in saturation level is quite slow and almost imperceptible – which could justify the presence of RERAs, especially if a resaturation occurs at the end of algorithm's measurement. It is also possible that PAT and heart rate response values of a patient with severe OSAS are different than those values of a patient with mild OSAS during an apnea episode. Furthermore, it can be observed that the resaturation rate can vary significantly in the same patient during different time segments.21 This fact does not diminish the portable device usefulness, as clinical data associated with a prior screening procedure result in high sensitivity in the diagnosis of OSAS, obviating the exclusion of potential patients. It can be inferred that the accuracy of this portable monitor is better for patients with high probability of having moderate or severe OSAS. However, in the case of patients under suspicion of a different sleep disorder than DRS, laboratory polysomnography must be indicated as a diagnostic method.22,23
According to a recent meta-analysis published by Yalamanchali et al., a high correlation of RDI and AHI (r=0.889 [95% CI, 0.862–0.911], p<0.001) was demonstrated. Studies comparing RDI between PAT and PSG had a combined correlation of 0.879 (95% CI, 0.849–0.904, p<0.001), while in those comparing AHI, this correlation was 0.893 (0.857–0.920, p<0.001). On the other hand, in those studies that compared the oxygen desaturation index (ODI), a correlation of 0.942 (0.894–0.969, p<0.001) was found.24 An study on children showed that PAT sign was significantly higher in those subjects with OSAS compared with controls, indicating increased sympathetic tone during wakefulness.25 Arterial stiffness also correlated with sleep disorders (arousal index) and mean O2 saturation; this variable better reflects the cardiovascular impact of OSAS vs. AHI in conventional PSG. Increased arterial stiffness (AS) is widely recognized as a determinant factor of cardiovascular risk and has also been linked to OSAS.26
There are some limitations to this study. The evaluation of arterial tonometry was performed in a sleep laboratory, and not in the patient's home. The use of this portable device is suitable for home monitoring; thus, further studies should be conducted in order to evaluate the reproducibility of these results outside the sleep laboratory. However, a scheme of carrying out both tests simultaneously would exclude the possible variation of results that could occur from one night to the next. Another limitation is the small sample, and there was no control group without signs and symptoms of obstructive sleep apnea. More studies should be conducted with a larger number of individuals.
ConclusionRespiratory parameters derived from arterial tonometry showed a good correlation and accuracy when compared with polysomnography; and AHI>20/h showed better sensitivity and specificity. Peripheral arterial tonometry is a very valuable option for the diagnosis of OSAS, especially in cases of patients with high probability of moderate or severe OSAS. Because this is an easy-to-use portable device, the results are readily provided and interpreted; thus, this would serve as an option to optimize the identification of patients with suspected OSAS. In this manner, its use would allow a more prompt treatment of this disease.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Pinto JA, de Godoy LBM, Ribeiro RC, Mizoguchi EI, Hirsch LAM, Gomes LM. Accuracy of peripheral arterial tonometry in the diagnosis of obstructive sleep apnea. Braz J Otorhinolaryngol. 2015;81:473–8.
Institution: Otorhinolaryngology, Head and Neck Surgery and Sleep Medicine Center of São Paulo (NOSP), São Paulo, SP, Brazil.