Intrathecal fluorescein has been effective for topographic diagnosis of rhinoliquorrhea. Nonetheless, there are no reports on the study of cerebral spinal fluid (CSF) after use of intrathecal fluorescein.
ObjectiveA prospective study attempting to evaluate CSF through chemical and cytological analysis, after injection of fluorescein.
MethodsProspective analysis of 24 samples of CSF after intrathecal injection of fluorescein for topographic diagnosis of CSF fistulae, collected at the time of puncture and after 24 and 48h, divided by cellularity: Group 1, up to five cells, and Group 2, with more than five cells.
ResultsThe yellow-greenish color of CSF remained after 48h in 36%, evidencing permanence of fluorescein. No changes in protein and glucose levels were observed between 0–24h and 0–48h. In group 2, an increase in cell count was observed between 24h and 48h (p=0.019). In both groups, there was an increase of neutrophils between 0 and 48h (p=0.048) and a decrease between 24 and 48h (p=0.05).
ConclusionIntrathecal fluorescein provoked discreet meningeal reactions, such as an increase of cells between 24 and 48h and an increase of neutrophils at 24h, with a subsequent decrease at 48h with no correlation with symptomatology.
A fluoresceína intratecal tem sido efetiva no diagnóstico topográfico da rinoliquorréia. Entretanto, não há estudos no líquor após o uso de fluoresceína intratecal.
ObjetivoEstudo prospectivo visando avaliar o líquor, através de análise química e citológica, após injeção de fluoresceína.
MétodoAnálise prospectiva de 24 punções após injeção intratecal de fluoresceína para diagnóstico topográfico de fístula liquórica, coletado no momento da punção, 24 e 48 horas, divididos pela celularidade: grupo 1, com até 5 células e grupo 2 com mais de 5 células.
ResultadoA coloração amarelo-esverdeada do líquor permaneceu após 48 horas em 36%, evidenciando permanência de fluoresceína. Observou-se ausência de mudanças no nível de proteína e glicose entre 0–24 horas e 0–48 horas. No grupo 2, um aumento na contagem celular foi observado entre 24 e 48 horas (p=0,019). No dois grupos juntos, observou-se um aumento de neutrófilos entre 0 e 48 horas (p=0,048) e uma diminuição entre 24 e 28 horas (p=0,05).
ConclusãoFluoresceína intratecal provocou discretas reações meníngeas, como o aumento de células entre 24 e 48 horas e aumento dos neutrófilos em 24 horas com uma subsequente diminuição em 48 horas sem correlação com sintomas.
Cerebral spinal fluid (CSF) fistulae can occur from nose or external ear canal, and from a traumatic or surgical defect in skull base or spine. Fluid leakage is the result of dural and arachnoid laceration, with fistula formation. CSF rhinorrhea is classically defined as the presence of CSF in nasal cavity, which implies the existence of a bone and dural defect resulting in a communication between the subarachnoid space and the upper airway cavities.1 These situations require a precise diagnosis for their etiology and location,2,3 together with a suitable treatment, due to an increased risk of neurological complications, mainly bacterial meningitis.
Although there have been several advances in preoperative tests and in examinations to diagnose CSF rhinorrhea, the use of fluorescein, which began in the 1960s, has been proven effective as to detection of sites of CSF fistulae.4–9
However, reports of adverse reactions are few, and are mainly related to high concentrations of fluorescein; some centers are still hesitant to use this strategy routinely.10–18 In 2001, a fluorescein solution was described in order to facilitate and hasten the identification of fistula sites. Previous studies describing the use and safety of an intrathecal hypodense fluorescein solution have already been published, but they rarely evaluate the changes in CSF due to its use.
The objective of this study was to determine the changes in CSF after using a hypodense fluorescein solution. Changes in coloration, cellularity, and glucose and protein levels in CSF of patients submitted to its use were evaluated.
MethodsThis was a prospective study of 24 consecutive patients aged 3–54 years (mean, 31 years) submitted to an intrathecal injection of hypodense fluorescein solution for an endoscopic intraoperative diagnosis of CSF leakage from the anterior aspect of skull base.
All patients provided informed consent after a discussion of the risks, benefits, and alternatives. The study was approved by the Ethics Committee of the institution under No. ETIC 075/00.
Thirteen patients were male (54%) and 11 female (46%). The main cause of CSF rhinorrhea was a traumatic event in 14 patients (58%), spontaneous rhinorrhea in five patients (21%), and meningocele or meningoencephalocele in five patients (21%). The exclusion criterion was the occurrence of meningitis in a previous period of three months. No patients were excluded from this study.
All patients were submitted to a lumbar puncture under general anesthesia at the beginning of surgery. CSF was collected at this time for chemical and cytological analysis.
After CSF collection, if the patient weighed more than 50kg, 0.5mL of 5% fluorescein solution diluted with 10mL of distilled water was injected. If the patient weighted less than 50kg, or in children, 0.1mL/kg of the described solution was injected.19 This study used Fluidag® (sodium fluorescein, sodium bicarbonate, and phenylmercuric nitrate; Oftalmopharma – São Paulo, Brazil). Fludiag® consists of a solution of 5% sodium fluorescein dissolved in a physiologically compatible buffer containing sodium bicarbonate as alkalinizing agent in order to maintain the pH in an 8.0–9.8 range, 0.0001% phenylmercuric nitrate as preservative, and water for injection.
Soon after the injection, which was slowly performed over 2–3min, the patient was positioned supine, with his/her back slightly raised at approximately 30°; then, the surgery was initiated.
After surgery and with the CSF fistula endoscopically closed, a lumbar drainage catheter inserted for the injection of fluorescein was kept closed. CSF samples were collected at 24h and 48h after the procedure. These samples were submitted to chemical/cytological analysis.
Chemical analysis of CSF included color (green, yellow-green, or clear), and glucose and protein levels by a colorimetric method.
In the cytological analysis, total number of erythrocytes and nucleated cells (neutrophils, lymphocytes, monocytes, eosinophils, and basophils) was obtained. A Neubauer chamber was used for cytological analysis.
The patients were divided into two groups, according to their initial number of cells in CSF: Group 1 (up to five cells per mL) and Group 2 (more than five cells per mL). These groups were compared, with the aim to examine the difference between glucose and protein levels and cellularity.
The study design made no provision for a control group, that is, one without fluorescein injection.
All results were statistically analyzed using Pearson's coefficient, with statistical significance set at 95% (p<0.05).
ResultsIn all cases, hypodense fluorescein present in the endoscopic surgical field was detected, with successful closure of CSF fistula. There was no need to use special light filters. There were no complications, adverse reactions, or meningitis cases in the whole sample of 24 patients. Group 1 included 14 patients and Group 2, ten patients.
As to CSF staining, CSF samples collected before fluorescein injection were clear in all patients. At 24h, in 20 patients (80%) a predominantly yellow-green color was observed, indicating presence of fluorescein. At 48h, nine patients (36%) still exhibited a yellow-green color in their CSF samples.
As to glucose level, there was no statistically significant difference between the two groups accessed concomitantly at the zero-h (p=0.5), 24-h (p=0.24), or 48-h (p=0.5) measurements.
Regarding protein level, there was no statistically significant difference with respect to protein levels between the two groups analyzed concomitantly at zero-h (p=0.37) and 24-h (p=0.16) measurements. At the 48-h measurement, a statistically significant difference was observed, with elevation of protein levels in Group 1 (p=0.032).
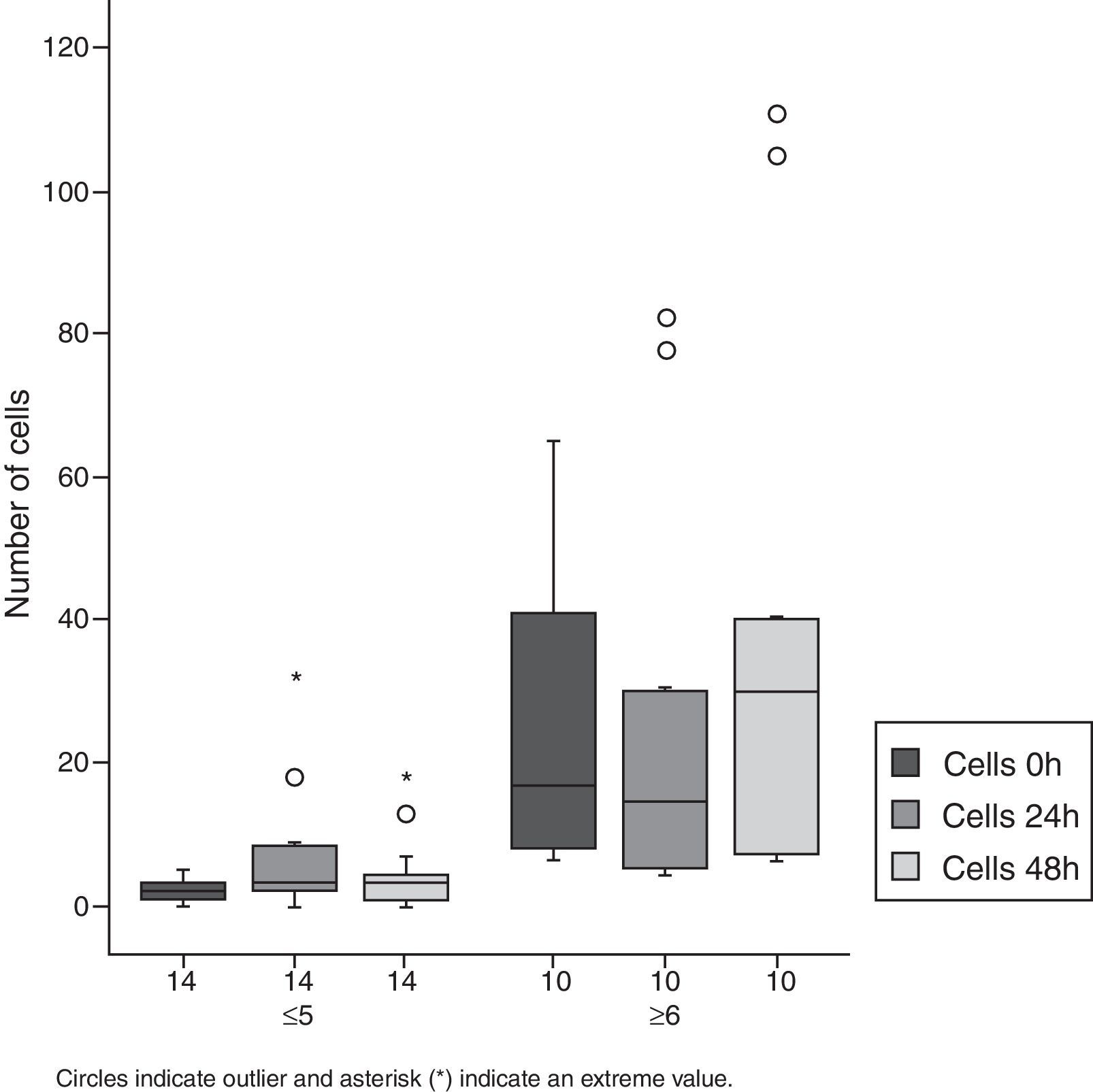
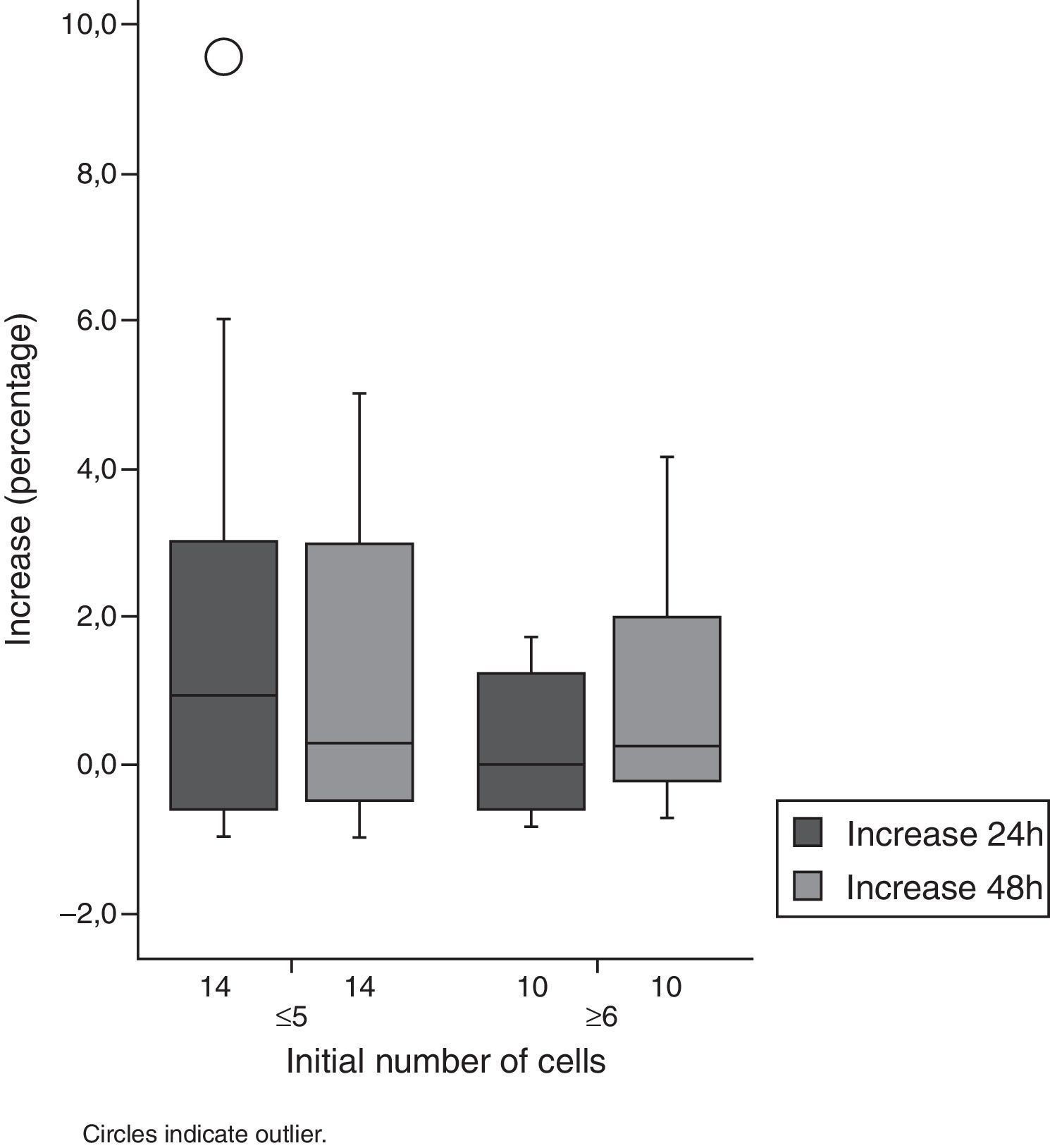
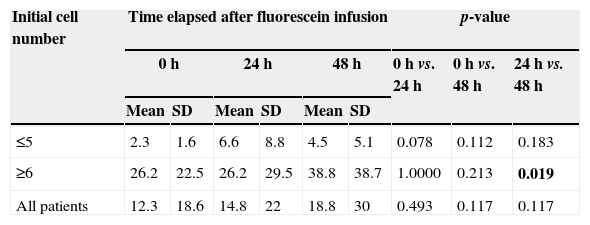
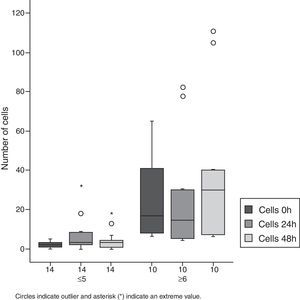
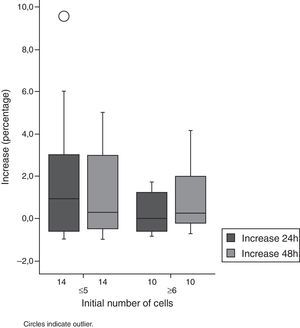
As to cell count, an increase in this parameter was also observed. In Group 1, almost all patients showed an increase of 600%, when compared to cell count baseline values. Half of the patients showed an increase in their cell counts in excess of 100% in both groups (Figs. 1 and 2, Table 1).
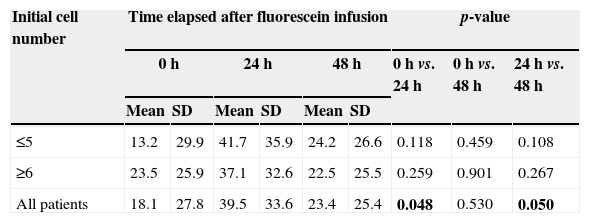
Number of cells according to time course and initial cell number.
| Initial cell number | Time elapsed after fluorescein infusion | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0h | 24h | 48h | 0h vs. 24h | 0h vs. 48h | 24h vs. 48h | ||||
| Mean | SD | Mean | SD | Mean | SD | ||||
| ≤5 | 2.3 | 1.6 | 6.6 | 8.8 | 4.5 | 5.1 | 0.078 | 0.112 | 0.183 |
| ≥6 | 26.2 | 22.5 | 26.2 | 29.5 | 38.8 | 38.7 | 1.0000 | 0.213 | 0.019 |
| All patients | 12.3 | 18.6 | 14.8 | 22 | 18.8 | 30 | 0.493 | 0.117 | 0.117 |
p-values <0.05 are in bold type.
Among Group 2 patients, a statistically significant increase in cell count was observed at 48h.
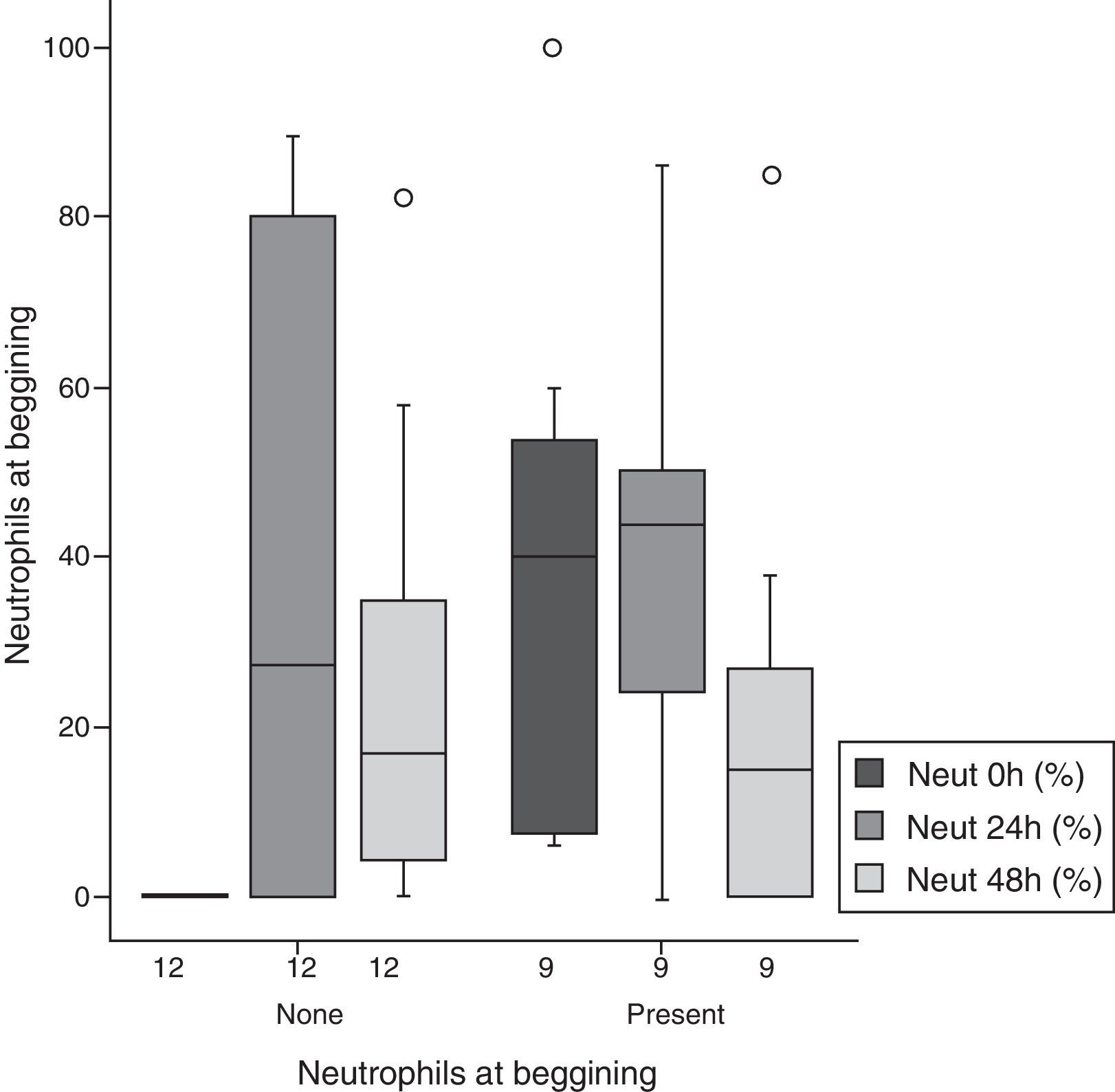
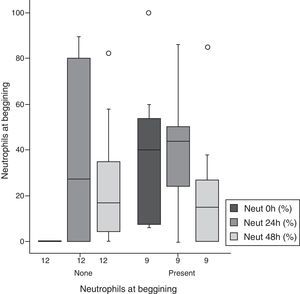
The percentage of neutrophils was similar for 24-h and 48-h measurements in both groups; compared to baseline values, an increase at 24h (p=0.048) and a decrease at 48h (p=0.05) were noted (Fig. 3, Table 2).
Percentage of neutrophils according to progression of time and initial cell number.
| Initial cell number | Time elapsed after fluorescein infusion | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0h | 24h | 48h | 0h vs. 24h | 0h vs. 48h | 24h vs. 48h | ||||
| Mean | SD | Mean | SD | Mean | SD | ||||
| ≤5 | 13.2 | 29.9 | 41.7 | 35.9 | 24.2 | 26.6 | 0.118 | 0.459 | 0.108 |
| ≥6 | 23.5 | 25.9 | 37.1 | 32.6 | 22.5 | 25.5 | 0.259 | 0.901 | 0.267 |
| All patients | 18.1 | 27.8 | 39.5 | 33.6 | 23.4 | 25.4 | 0.048 | 0.530 | 0.050 |
p-values <0.05 are in bold type.
Intrathecal CSF fluorescein staining has been previously described.6,7,12,15,16,19 This procedure allows CSF detection from blood or secretions present in the operating field. This justifies the absence of a control group, i.e. without application of fluorescein.
Intrathecal application of fluorescein is an off-label use of this product and its use requires documentation and a specific discussion with the patient, requiring informed written consent.8 Several adverse events such as seizures, transient paralysis, and neuropathic pain have occasionally been attributed to its use in literature.11,12,15 The incidence of complications remains extremely low and some authors reported higher percentages in patients who received higher or more concentrated doses than that described in this study.15–19 The pathophysiology of these events remains unclear, but they may be secondary to a meningeal inflammation, which changes the chemistry of normal CSF and its cytology.8,12,15
Normal CSF has a protein content of about 20–45mg/dL. The glucose level in this medium is about 50–100mg/dL; CSF may contain up to five cells per mL. These were the values used in this study, in order to perform this evaluation.20
No adverse reactions were observed with the use of an intrathecal hypodense fluorescein solution at the dosage described in this study, even in children. With this dosage, the skull base defect visualization was considered effective.16,19
Nevertheless, there were chemical and cytological changes in CSF. In 36% of patients, CSF color, which was clear in the first collection, changed with the use of fluorescein, up to 48h after injection. This observation can be correlated with the findings of cell progression and percentage of neutrophils.
The presence of fluorescein in CSF can act as a triggering factor for neutrophilia and increased cell count, as well as to their subsequent decrease after the fluorescein levels in CSF diminished.
Although a lumbar drainage catheter (used only for CSF collection) remained in all patients, it cannot be assumed that these reactions occurred only due to the presence of this device, which was maintained for 48h. In that case, the catheter would increase the cell count in all patients, in a direct relation to the exposure time – a fact that was not observed.20 However, the possibility that phenylmercuric nitrate and sodium bicarbonate may cause some of the changes observed in this study cannot be ruled out. Nonetheless, the cytological changes observed were minimal, and were not associated with side effects. Besides, these changes can be even smaller, depending on the presence of these compounds in the solution used.
There were no significant changes in glucose levels in CSF. This finding is important, since none of the patients showed any symptom of bacterial meningitis during the study. On the other hand, protein levels from Group 1 patients increased at the 24-h and 48-h (p=0.032) measurements vs. Group 2 patients, which can be explained by the increased number of cells present in the CSF samples of Group 1.
ConclusionBased on these results, it can be concluded that the intrathecal hypodense fluorescein solution (Fluidag®) promotes chemical and cytological changes in CSF, with an increase in cell number (mainly at 24h and 48h) and in neutrophils at 24h with subsequent decrease at 48h. Protein levels increased at 24h and 48h. There were no changes in glucose levels. Nevertheless, these changes did not translate into any clinical manifestations.
Conflicts of interestsThe authors declare no conflicts of interest.
Please cite this article as: Guimarães RES, Stamm AEC, Giannetti AV, Crosara PFTB, Becker CG, Becker HMG. Chemical and cytological analysis of cerebral spinal fluid after intrathecal injection of hypodense fluorescein. Braz J Otorhinolaryngol. 2015;81:549–53.
Institution: Faculdade de Medicina, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, Minas Gerais, MG, Brazil.