Mismatch negativity, an electrophysiological measure, evaluates the brain's capacity to discriminate sounds, regardless of attentional and behavioral capacity. Thus, this auditory event-related potential is promising in the study of the neurophysiological basis underlying auditory processing.
ObjectiveTo investigate complex acoustic signals (speech) encoded in the auditory nervous system of children with specific language impairment and compare with children with auditory processing disorders and typical development through the mismatch negativity paradigm.
MethodsIt was a prospective study. 75 children (6–12 years) participated in this study: 25 children with specific language impairment, 25 with auditory processing disorders, and 25 with typical development. Mismatch negativity was obtained by subtracting from the waves obtained by the stimuli /ga/ (frequent) and /da/ (rare). Measures of mismatch negativity latency and two amplitude measures were analyzed.
ResultsIt was possible to verify an absence of mismatch negativity in 16% children with specific language impairment and 24% children with auditory processing disorders. In the comparative analysis, auditory processing disorders and specific language impairment showed higher latency values and lower amplitude values compared to typical development.
ConclusionThese data demonstrate changes in the automatic discrimination of crucial acoustic components of speech sounds in children with specific language impairment and auditory processing disorders. It could indicate problems in physiological processes responsible for ensuring the discrimination of acoustic contrasts in pre-attentional and pre-conscious levels, contributing to poor perception.
Mismatch Negativity (MMN), uma medida eletrofisiológica, mede a habilidade do cérebro em discriminar sons, independente da capacidade atencional e comportamental. Assim, esse potencial mostra-se promissor no estudo das bases neurofisiológicas que subjaz o processamento auditivo.
ObjetivoInvestigar a discriminação de sinais acústicos complexos (fala) no sistema auditivo por meio do MMN, com crianças com distúrbio específico de linguagem (DEL), comparando com transtorno do processamento auditivo (TPA) e desenvolvimento típico (DT).
MétodoEstudo Prospectivo. 75 crianças (6-12 anos) participaram deste estudo: 25 crianças com DEL, 25 com TPA e 25 em DT. O MMN foi obtido por meio da subtração das ondas obtidas pelos estímulos/ga/(frequente) e/da/(raro). Foram analisadas as medidas de latência do MMN e duas medidas de amplitude.
ResultadosFoi possível verificar ausência do MMN em 16% no TPA e 24% DEL. Na análise comparativa, os grupos TPA e DEL apresentaram maiores valores latências e menores valores de amplitude em relação ao DT.
ConclusãoEstes dados demonstram uma alteração na discriminação automática de componentes acústicos cruciais dos sons de fala em crianças com TPA e DEL, o que poderia indicar alterações nos processos fisiológicos responsáveis pela discriminação precisa de contrastes acústicos em níveis pré-atencionais e pré-conscientes, contribuindo para uma percepção deficiente.
Abnormalities in auditory temporal processing have been one of the main theories used to try to explain the etiology of language development disorders. This theory suggests that one of the causes for language development disorders (among them, specific language impairment [SLI]) is related to changes in the ability to process sounds and abnormalities in neural coding of auditory information,1–3 contributing to changes in the perception of fundamental acoustic cues in speech sound signals.
Despite nearly a century of research, no consensus has yet been reached on the physiological basis of causality concerning this language development disorder, as the results of studies have failed to find evidence of changes in the auditory processing of children with SLI.4,5 Therefore, the etiological causes of language development disorders still remain controversial.
Although studies using behavioral measures have shown inconsistent results, electrophysiological evaluations been proven to be ideal for investigating the neural bases of speech perception; they due not interfere with the subjective behavioral response and are independent of it and they are useful in establishing anatomical and functional associations in the human auditory system.6
Mismatch negativity (MMN) is an electrophysiological measure that reflects the brain's capacity to discriminate sounds, regardless of the individual's attentional and behavioral capacity. Initially described by Näätänen et al.7 (1978), MMN is a cortical evoked potential that is detectable when a change occurs in the middle of a sequence of repeated acoustic stimuli.8,9
Characterized by a negative deflection that occurs after the P2 response, MMN usually occurs between 150 and 250ms after the stimulus presentation, with latency and amplitude varying, depending on the stimulus.10–12
Most studies use simple paradigms, in which frequent and infrequent stimuli (e.g., 1000Hz and 1100Hz tones, respectively) are presented in an oddball paradigm, similar to that used for the P300, with the infrequent stimulus eliciting MMN.7,10,12,13
However, MMN can also be elicited by changes in complex stimuli such as speech sounds.14–18 The speech signal consists of harmonically rich elements that change rapidly with respect to frequency. This complex, spectrum-temporal structure requires neural integrity for accurate coding of its signal.19 The acoustic properties of speech sounds are encoded at all levels of the auditory system and these acoustic parameters are represented differently along the auditory pathway. Additionally, there is evidence that they are probably modified at each level of the auditory nerve pathway.20 Thus, simultaneous and coordinated activation of large and different populations of neurons is required for speech processing and understanding, from the eighth cranial nerve signal transduction to the cortex.19
Based on previously established associations, this study aimed to assess the discrimination of complex acoustic signals (speech) in the auditory system through MMN in individuals with specific language impairment (SLI), compared to children with auditory processing disorder (APD) and typical development (TD).
MethodsThis study was approved by the Research Ethics Committee, Protocol #1049/07. Parents or guardians were instructed regarding the study procedures and signed an informed consent.
SampleA total of 75 children were assessed, aged between 6 and 12 years, in a teaching and research center at a university in São Paulo, Brazil. All individuals had hearing thresholds within the normal range (≤15dB HL) for the assessed frequencies (500–4000Hz); speech recognition scores were >88%; normal tympanometric measures were seen; and also an absence of neurological, cognitive, and psychiatric disorders. The individuals were divided into three groups:
- a)
TD group: 25 children with typical development, according to information obtained through interviews with the parents/guardians and teachers, with absence of school difficulties, speech, and language disorders. In addition, these children had normal performance at the auditory processing assessment.
- b)
APD group: 25 children diagnosed with APD at a laboratory of audiological investigation of auditory processing located in the same place where the study was conducted. The APD diagnosis was achieved using criteria established by the American Speech-Language-Hearing Association (ASHA), i.e. performance below normal for the age in at least two tests of the auditory processing assessment test battery. The minimum test battery applied in this group and the TD group consisted of temporal processing tests (test of frequency and/or duration pattern) and monotic (speech with noise and/or picture with noise), and dichotic listening (dichotic digit test and/or Staggered Spondaic Words [SSW]).
- c)
SLI group: 25 children diagnosed with SLI using the international reference criteria,1 namely: persistent speech and/or language difficulty in the absence of hearing loss; changes in cognitive development; speech motor development impairment; comprehensive developmental disorders, syndromes, and sensorineural changes; and acquired neurological lesions.21,22 These children were diagnosed and underwent speech therapy in a specific laboratory in the same location where the research was conducted.
After the children were selected and submitted to hearing screening, children were asked to choose a movie (cartoon) to watch during the evaluation, with a presentation intensity of approximately 40dB (A).15,23 Some studies have found that individuals are more collaborative when they are watching a video during the evaluation sessions.24,25
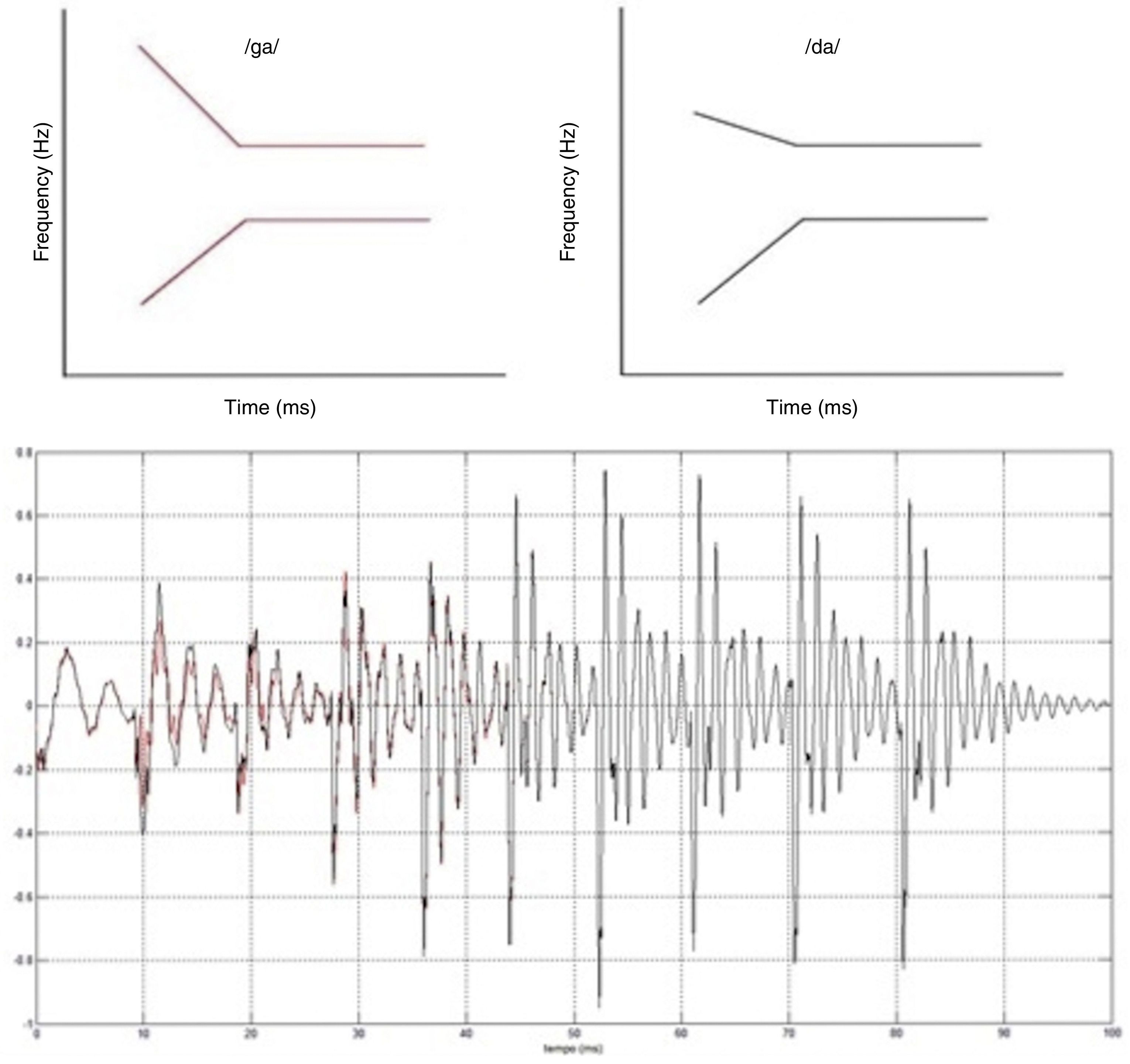
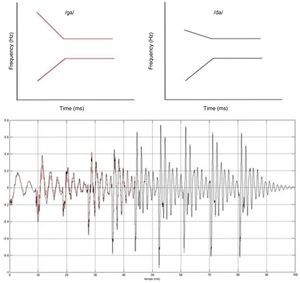
The event-related auditory evoked potential (MMN) was used to investigate mechanisms that permeate auditory sensory discrimination. MMN was obtained by presenting acoustic speech stimuli – plosive consonants /da/ and /ga/. The speech stimuli were synthesized26 with 48kHz, of 16 bit, and length of 100ms. The stimuli consisted of five formants, differentiated at the onset frequencies in the transition from the second to the third formant (Fig. 1).
Stimuli were presented at 75dB intensity NNA with analysis time of 500ms, sensitivity of 100μV, and 1–30Hz filter (with off-line filter from 1 to 15Hz). Approximately 1600 stimuli were used, of which 1400 (86%) were common (/ga/) and 200 (14%) rare (/da/), presented in eight sets of 200 stimuli (175 frequent and 25 rare), with a four-second interval between the sets. The stimuli were randomly presented (oddball paradigm) to trigger MMN, at a rate of 1.5 stimuli per second. The stimuli were presented in the right ear, through insert earphones because there is evidence of a right ear advantage for the processing of speech sounds.24
The tracings were obtained through an electroneuromyograph, model Navigator Pro (Biologic Systems Corporation; Natus Medical Inc – Mundelein, United States).
As the MMN, by definition, is elicited only by a deviant or rare stimulus, the trace was obtained as follows: by subtracting the mean of the tracings corresponding to the stimulus /da/ presented in oddball paradigm /da/ (rare) from the mean of the tracings obtained in response to the stimulus /ga/ (frequent).15 The MMN was identified as the wave of negative polarity and with approximate latency of 150–250ms post-stimulus (Näätänen et al.12) and was captured by the electrodes in positions Fz, M2 (right mastoid) and with the Fpz as the ground wire.27
Amplitude measurements were analyzed and calculated by placing one of the reference cursors on the negative polarity point (MMN) and the other cursor on the positive point previous to MLM to establish the on-MMN amplitude and posterior to MMN to identify the off-MMN amplitude.
Statistical analysisAccording to previously specified objectives, the statistical method aimed to compare the groups regarding the discrimination of complex acoustic signals (speech) in the auditory system through the MMN electrophysiological assessment. For this purpose, descriptive analyses were performed of the values obtained in the variables resulting from MMN – latency (described in milliseconds – ms) and amplitude (described in microvolts – μV) – by building tables with the values observed in the descriptive statistics: mean, standard deviation, minimum, median, and maximum. To compare the test means in the three groups, analysis of variance (ANOVA) was applied. The significance level was set at 0.05 and significant items were identified with an asterisk (*). When necessary, Tukey's multiple comparison method was used for further analysis.
To complement the descriptive analysis, a 95% confidence interval (95% CI) was used to assess variance of the mean. The significance level was set at 5%.
ResultsAll individuals in the TD group exhibited an MMN, response whereas only 84% of individuals in the APD group (21/25 subjects) and 76% of the SLI group (19/25 subjects) showed the response. Therefore, individuals that did not respond to MMN were excluded from the subsequent analysis.
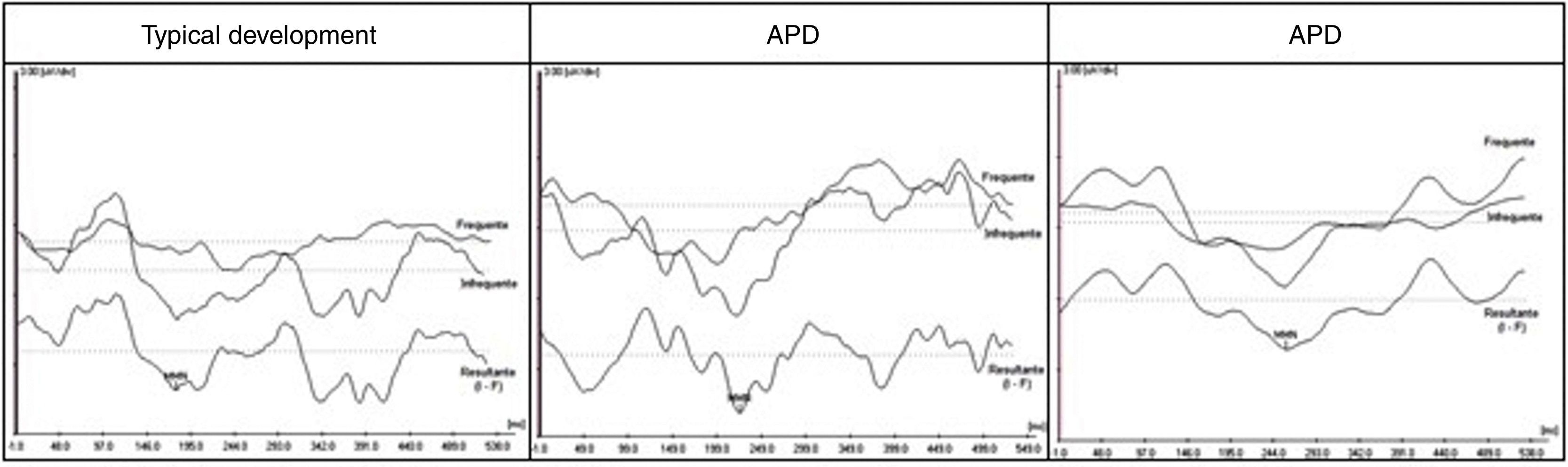
Fig. 2 shows the tracings obtained by MMN in an individual from each group (TD, APD, and SLI).
Mismatch negativity (MMN) tracings for the contrasts of /ga/ (frequent) and /da/ (rare) stimuli and that observed (rare-frequent) in an individual with typical development (TD), an individual with APD, and another with SLI. APD, auditory processing disorder; SLI, specific language impairment.
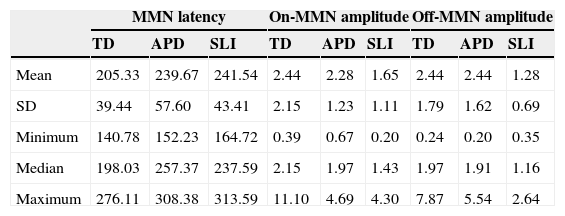
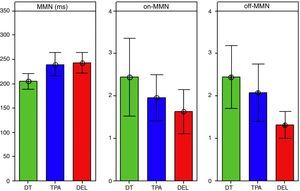
Table 1 shows descriptive statistics of the data obtained by the three groups. It can be observed that the mean MMN latency in the TD group was shorter than values observed in the APD and SLI groups. The median values show that 50% of the APD group had latency values for MMN > 257.37ms, i.e. above the value of 250ms proposed by Näätänen et al.12
Descriptive statistics for the mismatch negativity (MMN) response values, considering the measures of latency and amplitude for all three groups.
| MMN latency | On-MMN amplitude | Off-MMN amplitude | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TD | APD | SLI | TD | APD | SLI | TD | APD | SLI | |
| Mean | 205.33 | 239.67 | 241.54 | 2.44 | 2.28 | 1.65 | 2.44 | 2.44 | 1.28 |
| SD | 39.44 | 57.60 | 43.41 | 2.15 | 1.23 | 1.11 | 1.79 | 1.62 | 0.69 |
| Minimum | 140.78 | 152.23 | 164.72 | 0.39 | 0.67 | 0.20 | 0.24 | 0.20 | 0.35 |
| Median | 198.03 | 257.37 | 237.59 | 2.15 | 1.97 | 1.43 | 1.97 | 1.91 | 1.16 |
| Maximum | 276.11 | 308.38 | 313.59 | 11.10 | 4.69 | 4.30 | 7.87 | 5.54 | 2.64 |
For the measurement of on-MMN amplitude, the TD group showed higher amplitude values, when compared to the APD and SLI groups. However, for the off-MMN amplitude, values observed in the TD and APD groups were very similar.
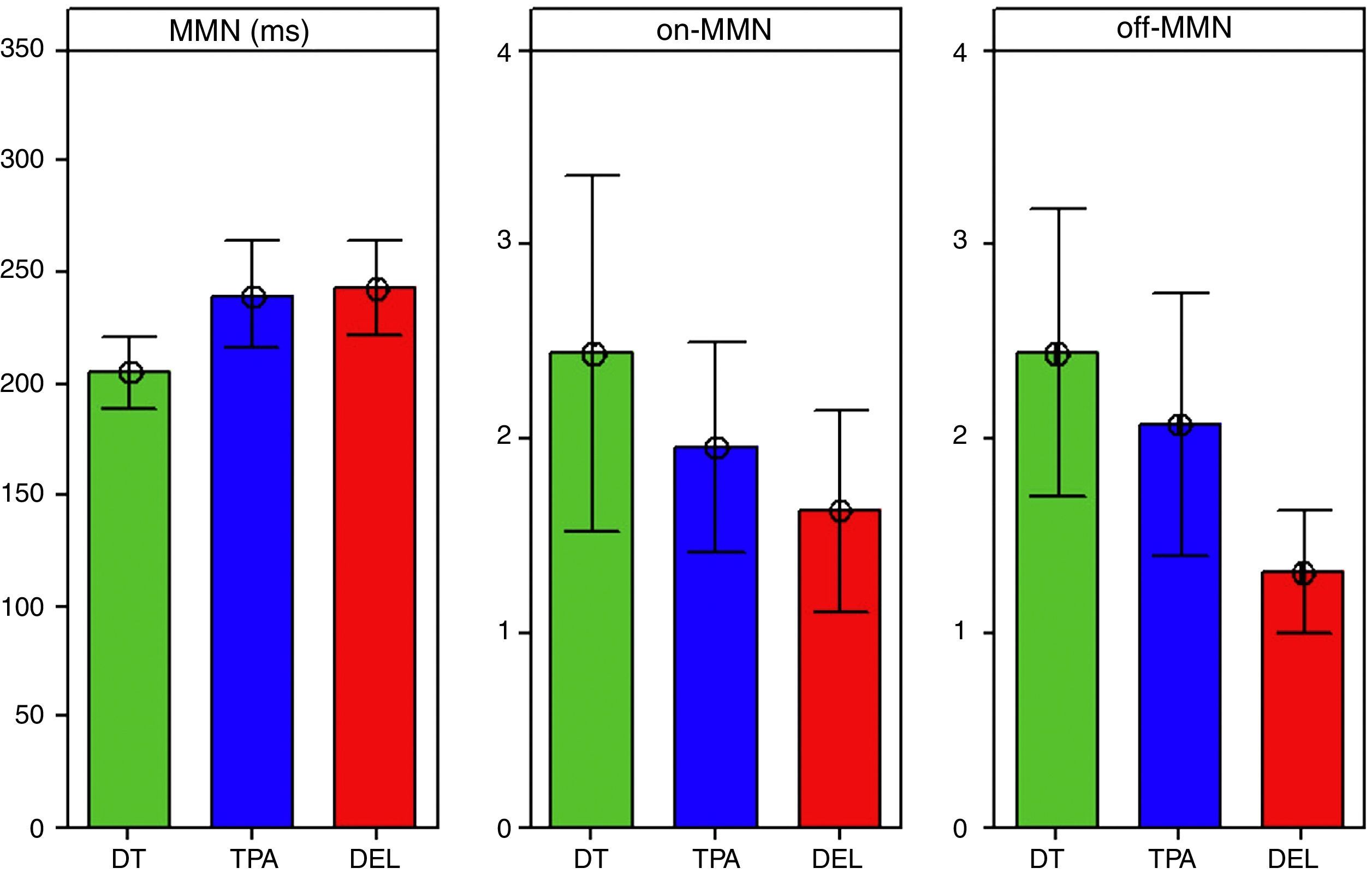
Fig. 3 shows the bar graph with confidence interval (95%) of the means and level of significance when comparing the means of the three groups for latency and amplitude measurements.
For the mean values of MMN latency, a statistically significant difference was observed between groups (F [2.62]=4.88, p=0.01*). Using Tukey's post hoc test, we verified that this significance was observed in the comparisons between APD and TD (p=0.03*) and SLI and TD groups (p=0.02*).
Regarding the amplitude measurement, although the TD group showed higher amplitudes for both the on-MMN and off-MMN amplitudes, the error bar chart indicated a statistically significant difference when comparing the groups only for the off-MMN amplitude (F [2.62]=3.45; p=0.03). The Tukey's post hoc test verified that this significance only occurred in the comparisons between TD and SLI groups (p=0.03*).
DiscussionBased on our results, MMN elicited by small acoustic differences in speech sounds (/da/ and /ga/) was clearly present in all children in the TD group. This is consistent with previous studies that show a robust MMN in normal subjects.28 In other words, children from the TD group are capable of differentiating the stimuli, in oddball paradigm, regardless of attentional activities.
However, in the SLI and APD groups, the MMN was not elicited in all individuals. Furthermore, higher latency values and lower amplitudes were observed in the APD and SLI groups, compared to the TD group (Table 1 and Fig. 2). This may mean that both the APD and the SLI groups showed some impediment at neural levels to accurately discriminate the contrasts from the stimuli.
Therefore, this potential also indicates that phonological deficits can coexist with difficulties in processing acoustic differences between stimuli.
The reduction and absence of the MMN response that was observed only in the population with changes in this study could contribute to the impairment of perception and acoustic representation at pre-attentional and pre-conscious levels of this population.
According to Uwer et al.,29 children with SLI show specific deficits in automatic discrimination between consonant- and vowel syllables that differ between points of articulation. Davids et al.30 added that children with SLI have difficulties in processing non-verbal stimuli and that may coincide with phonological deficits.
These studies, together with other studies in children with language disorders,16,17,29,31–33 have found a decrease in spectrum contrast coding, expressed as the MMN change in this population, corroborating the findings of the present study.
On the contrary, some studies have not found the same MMN results for children with SLI.34,35 These studies found no abnormal responses to MMN in children with SLI, compared to children with typical development, both for speech stimuli and for non-verbal stimuli (tone burst). In addition to these aforementioned authors, Roggia and Colares36 studied MMN with non-verbal stimuli in children with APD. The authors also failed to find differences between the APD group and normal children.
Even though MMN is described in the literature as an important tool in the investigation of changes in the auditory processing of acoustic stimuli, few studies have used this potential in children with APD. We found no studies in the literature that simultaneously assessed the performance of children with APD and SLI using this potential. This fact can be explained by the difficulties in finding sufficient numbers of children with isolated APD, not associated with reading and language problems; there is also some debate on the diagnosis of APD.37
According to results obtained in this study, we found that the APD and SLI groups exhibited higher latencies and lower amplitudes and a higher percentage of absent MMN compared to the TD group, but the APD and SLI groups had similar performances regarding the MMN, and there were no statistically significant differences between the two groups.
Some studies show that changes in the discrimination of small acoustic differences are common characteristics in individuals diagnosed with APD and SLI. MMN amplitude has been associated with auditory perceptual measures and the absence of MMN indicates incapacity to perceive any difference between the sounds.38 Other important aspects that can influence MMN are the changes in short-term memory,12 and also alterations in long-term memory.8 Therefore, the changes found in MMN in this study were expected, as the above-mentioned factors are often altered, both for children with APD and for children with SLI.
Altered MMN responses have typically been attributed to changes in the auditory cortex. However, this cannot be affirmed, considering the present results indicate that changes in MMN lead to behavioral deficits observed in the APD and SLI groups, as there is evidence of interactions between physical characteristics of the stimulus and cognitive operations. Moreover, it was not possible to determine whether the changes found in MMN, both in the group with APD and in the group with SLI, have the same origin.
There is evidence that encoded stimulation changes are represented differently in the brain. Kraus et al.39 found more robust MMN in response to the /bad/–/wa/ stimuli (in which the difference is the duration between the formants), when compared to the /ga/–/da/ stimuli (in which the difference lies in the transition between the frequency of the second to the third formant). The authors defend the hypothesis that the regions that contribute to the formation of MMN vary according to the difference between the stimuli used.40
Thus, other studies using MMN with different stimuli should be employed in the study of children with APD and SLI in order to investigate possible similarities and differences between these changes, and to study the reason why only some of the children with alterations in auditory processing develop language disorders.
Another hypothesis that cannot be ruled out is the possibility that children with APD and SLI have a maturational delay in the overall development of the central nervous system. This hypothesis states that the electrophysiological differences in auditory responses seen between children with APD and SLI and normal children would indicate neurodevelopmental immaturity.41,42 It is known that the myelination process continues through childhood,43 and that these changes in auditory processing would reflect cortical development maturational delay. In spite of the consistency, some authors criticize this hypothesis based on studies of changes in children with language and learning disorders that persist even after adolescence until adulthood.44 Therefore, more longitudinal studies, particularly for speech sounds, are needed to determine whether this hypothesis is plausible.
The MMN in the present study appeared to reflect the neural response to changes in stimuli, that had been employed in other studies to investigate the auditory cortical activity associated with extraction of phonetic information from acoustic stimuli, information essential for word recognition. Thus, the MMN is an appropriate tool for the evaluation of speech perception that specifically requires the ability to encode dynamic changes in acoustic signals. Another advantage is that the MMN appears to precede linguistic and cognitive processing at this processing level.40
Considering the various hypotheses, it is difficult to conclude which factors are responsible for the changes found in MMN and, also, whether these factors are similarly manifested in the SLI and APD groups. However, the present study showed that, using the event-related MMN potential, it was possible to study auditory processing for discrimination of acoustic events independent of any behavioral response, as some children have attentional problems,45 or problems in language expression and/or reception, that can influence performance at behavioral tests, commonly used to assess auditory processing.
ConclusionMMN allowed the study of acoustic signal discrimination in children with typical performance and with language and auditory processing disorders. Moreover, these findings showed evidence of changes in pre-attentional discrimination of acoustic contrasts, both in children with APD and SLI. These results suggest that this alteration in the automatic cortical change detection process is similar between children with APD and SLI.
FundingThis study was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP, Process number (2009/18417-0).
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Rocha-Muniz CN, Lopes DMB, Schochat E. Mismatch negativity in children with specific language impairment and auditory processing disorder. Braz J Otorhinolaryngol. 2015;81:408–15.