The literature indicates that neonatal hearing screening should be universal, so a description of programs that adopt this recommendation is relevant.
ObjectiveTo describe the results of newborn hearing screening and the profile of mothers and newborns attended to in a low-risk maternity setting, and to correlate the characteristics of this population with the results of transient evoked otoacoustic emissions.
MethodsA contemporary cross-sectional cohort study. The sample consisted of 670 infants and the procedures performed were audiological history, transient-evoked otoacoustic emissions (TEOAE), distortion product-evoked otoacoustic emissions (DPEOAE), and automated-brainstem auditory evoked potential (ABSAEP).
ResultsThe rate of success in this program was 98.5%, the failure rate was 0.62%, and that of non-attendance to finalize the diagnostic process, 0.93%. When correlating the variables studied with the results of transient evoked otoacoustic emissions, there was a significant negative correlation only for age of infant.
ConclusionThe program of this maternity hospital was effective and complies with national and international recommendations. The population consisted of young mothers with few pregnancy complications and healthy infants. The only variable that influenced transient evoked otoacoustic emission results, after hospital discharge, was the age at which infants were evaluated.
A literatura relata que a triagem auditiva neonatal deve ser universal, o que torna relevante a descrição de programas que adotam esta recomendação.
ObjetivoDescrever os resultados da triagem auditiva neonatal e o perfil das mães e recém-nascidos atendidos em uma maternidade de baixo risco e correlacionar as características desta população com os resultados das emissões otoacústicas evocadas transientes.
MétodoEstudo coorte contemporâneo com corte transversal. A amostra foi composta por 670 bebês e os procedimentos realizados foram: anamnese audiológica, emissões otoacústicas (EOA) transientes, EOA produto de distorção, e potencial evocado auditivo de tronco encefálico automático.
ResultadosO índice de passa neste programa foi de 98,5%; de falha de 0,62% e o de não comparecimento para finalização do processo diagnóstico de 0,93%. Ao correlacionar as variáveis estudadas com os resultados das emissões otoacústicas transiente houve correlação negativa significante apenas para a idade do bebê.
ConclusãoO programa desta maternidade mostrou-se efetivo e atende a recomendações nacionais e internacionais. A população foi composta por mães jovens com poucas intercorrências gestacionais e bebês saudáveis. A única variável que influenciou nos resultados das emissões otoacústicas por transiente, após a alta hospitalar, foi à idade em que os bebês foram avaliados.
The Universal Newborn Hearing Screening (UNHS) program seeks early detection of hearing loss, with the aim of evaluating the hearing ability of neonates with and without risk factors for hearing loss (RFHL). This process consists of performing behavioral, electroacoustic, and/or electrophysiological procedures to identify hearing loss.1
Discussions about the importance and implementation of newborn hearing screening programs were initiated in the 1990s. In 2000, the Brazilian Speech Therapy Council issued an opinion indicating the need to implement hearing screening procedures in neonates using some objective methodology already described in the literature, such as recording evoked otoacoustic emissions and brainstem auditory evoked potential (BAEP).2
Several local and state laws have been passed in this country, making completion of the UNHS compulsory in maternity wards. Of note is National Law No. 12,303 of August 2, 2010, which determines the obligation to carry out evoked otoacoustic emission tests in all hospitals and maternity wards in children born on their premises.3 However, it is known that few public maternity facilities run a systematic universal newborn hearing screening program.4
The literature reports that the most widely used methods in newborn hearing screening programs are probably the transient-evoked otoacoustic emission (T-EOAE) test in a first stage, and the auditory brainstem response in a second stage, for those infants who failed the T-EOAE test. The combination of both tests was designed to reduce the number of false-negative results, especially in cases of auditory neuropathy/dyssynchrony, in addition to improving the sensitivity and specificity of UNHS results.5–17
In reviewing the studies published in the Brazilian literature describing characteristics of newborn hearing screening programs, it is observed that most of them specify test results, gender, age, birth weight, and risk indicators. There have been numerous reports of screening results according to risk indicators.18–28
Unlike articles published in the literature, the present study aimed to describe a newborn hearing screening program in which the majority of treated newborns had no risk indicators for hearing loss, which would decrease the incidence of hearing loss in this population. In addition, it was intended to expand the description of the characteristics commonly reported in the literature for mothers and newborns.
Considering the above, the aim of this study was to describe the results of a newborn hearing screening program and the profile of mothers and newborns attended in a low-risk maternity ward, as well as to correlate the characteristics of this population with the results of transient evoked otoacoustic emissions.
MethodsThis was a contemporary cohort cross-sectional study, performed in a maternity hospital in São Paulo State and approved by the Institution's Research Ethics Committee, under No. 0703/2013.
This maternity ward is part of the Unified Health System (SUS) and cares for low-risk pregnant women, with an average of 1500 births per year. The institution is part of Rede Cegonha, a program developed by the Ministry of Health that offer humanized care with the following objectives: (1) implementation of a new model of health care for women and children, with a focus on delivery, birth, growth, and development; (2) Organization of maternal and child health services; and (3) reduction of maternal and infant mortality in the neonatal period. In addition to this program, this maternity ward has partnership with the Human Milk Bank of the city of Marília, São Paulo, Brazil in order to educate mothers on the importance of exclusive breastfeeding and addressing any problem related to breastfeeding and collection, storage, and donation of breast milk.
As to the composition of this sample, the following inclusion and exclusion criteria were used: signing the informed consent, screenings conducted in the period from May to November of 2013, and response of the mothers to anamnesis data.
Thus, this study was based on data from 670 newborns attended by this neonatal hearing screening program.
To achieve this goal, the following procedures were used: anamnesis, meatoscopy, and hearing tests (T-EOAE, distortion product-evoked otoacoustic emission [DP-EOAE], and automated-auditory brainstem emission potential [A-ABEP]).
Initially, an audiological history was obtained, based on a questionnaire (Annex) consisting of identification data, questions about gestational history, delivery, and newborn data, such as: gender, age, gestational time (preterm or term), mother's age, type of delivery (normal or cesarean), pregnancy complications, baby's birth weight, type of feeding, bottle feeding and/or pacifier use, and other risk factors for hearing loss,1 including phototherapy for hyperbilirubinemia. It was decided in favor of the addition of this indicator, because of the high incidence of hearing loss in children undergoing phototherapy in clinical practice.
Hearing procedures were divided into two stages: test and retest. For the test, T-EOAE was carried out; in case of failure in this test, DP-EOAE was added. For retest, T-EOAE, DP-EOAE, and A-ABEP were performed.
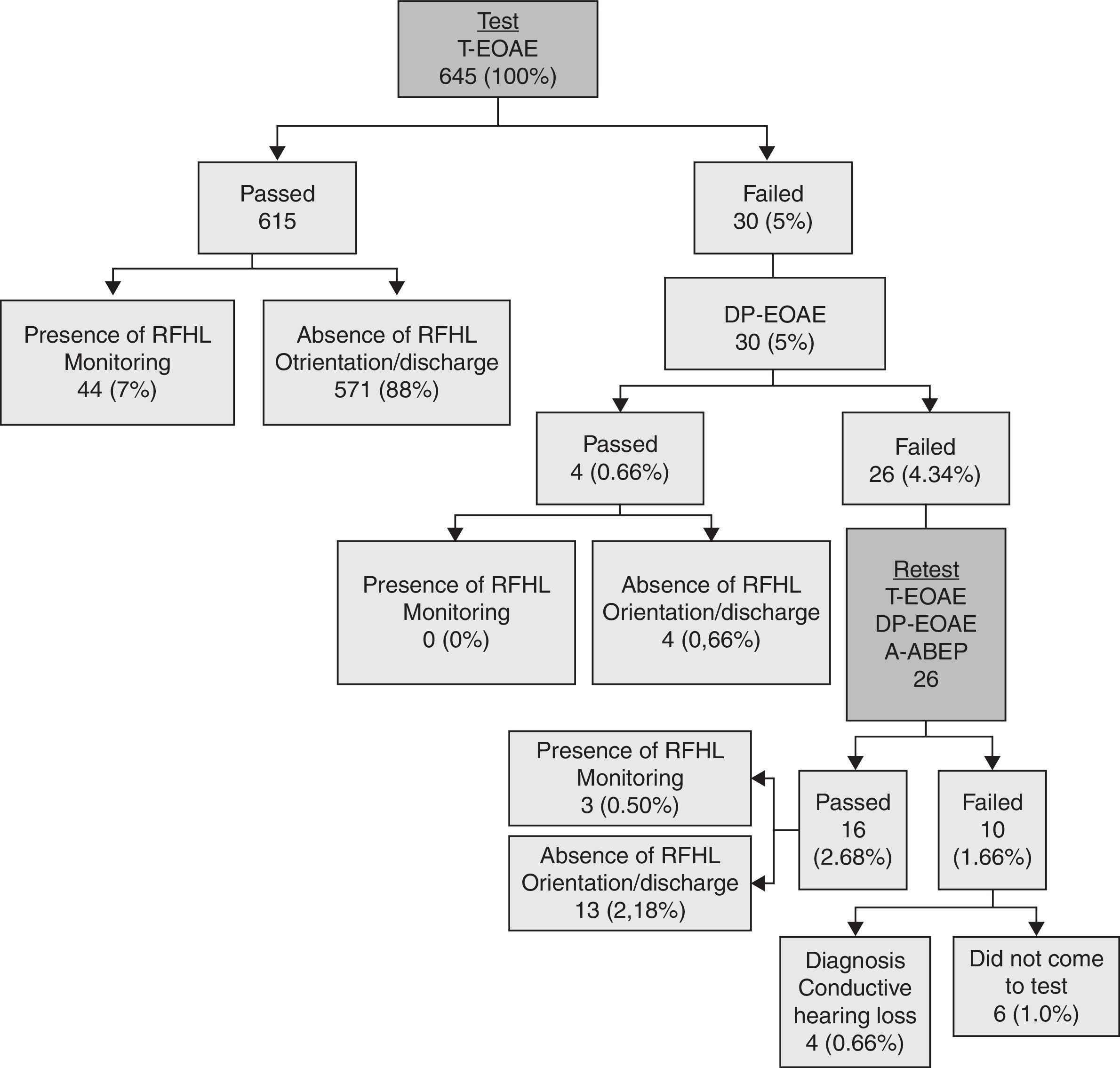
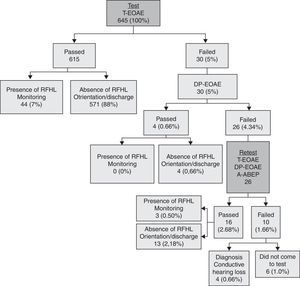
The flowchart in Fig. 1 describes in detail the hierarchy of steps in the second hearing screening, according to risk indicators.
In this program, the newborn was discharged from hospital and returned after about a week for hearing screening (test) and puerperal consultation. If the infant failed the test, a retest was scheduled to be performed in approximately 15 days. In the event of no attendance of the baby for the test or retest, the mother was contacted in order to schedule a new date.
It was considered that the baby passed the test when there were responses in both ears for the procedures performed.
It is noteworthy that in cases where newborns passed the hearing screening test and had no risk indicators for hearing loss, their parents received guidance about the typical of hearing and language development, and on how to proceed in case of any change in this development (i.e. a new assessment); then mother and baby were discharged. Newborns who passed the hearing screening test but showing risk indicators for hearing loss were referred for a monitoring program. In this program, the baby attended a consultation every two months during the first year of life, in order to evaluate and monitor its hearing and language development.
Infants who failed the hearing screening test were referred for a full hearing evaluation.
Evoked otoacoustic emission and automatic auditory brainstem evoked potential tests were carried out with the help of a hand-held AccuScreen® screener (Madsen), suitable for use in hearing screening programs. To capture the answers, an ear probe was coupled to the external ear of the newborn, preferably during physiological sleep, or when still and quiet. Before the procedures, an automatic calibration of the equipment was performed, which depended on the newborn's external auditory canal volume.
The transient evoked otoacoustic emissions were generated from a click-type stimulus (frequency range, 1.5–4.5kHz) with intensity ranging between 45 and 60dB HL. The minimum stability of the probe obtained during the test was 70%. For the analysis of results, the equipment counts response signal peaks; the presence of eight peaks was necessary to consider that the neonate passed the test.
The distortion product evoked otoacoustic emissions were generated from the presentation of a primary tone pair of different frequencies (F1 and F2) in a relationship F2/F1=1.22, in which F1 is the primary stimulus of lower frequency and F2 the primary stimulus of greater frequency; and the distortion product obtained will occur in a different frequency range. The stimuli were presented on two levels (L1/L2) of 60/50dB SPL. For screening and analysis of the results, protocol 1 of the equipment was used, which evaluates the frequencies of 5, 4, 3, and 2kHz, in this order. The test is completed when the newborn presents response in three frequencies (passed) or when it does not present response in two frequencies (failed).
The automatic brainstem auditory evoked potential test was conducted with the electrodes applied to vertex (active), zygoma (ground), and C7 vertebra (reference). For this purpose, Ambu® Neuroline 720 disposable electrodes were used. These devices were applied after skin cleaning with an abrasive paste (Nuprep®), ensuring 4Ω of maximum impedance for the electrodes. The stimulation parameters were: click sequence stimulus at 35dB nHL, sampling rate 16Hz, click level of approximately 80Hz, incoming bandwidth from 70Hz to 4kHz, and gain of 2000. For response analysis, the “passed” result was established when a response to the stimulus was detected by the machine.
The results of this study were presented with the use of descriptive and inferential statistics. Spearman's correlation was applied to verify the relationship among variables: birth weight, gender, age, gestational time, type of delivery, complications during pregnancy, Apgar score at 1 and 5min of life, risk indicators for hearing loss, and results of transient otoacoustic emissions. The significance level was set at 5% (p≤0.05).
It is noteworthy that the decision was made to correlate the variables only with the results of transient evoked otoacoustic emissions, since distortion product evoked otoacoustic emissions and automatic brainstem auditory evoked potentials were carried out only in infants that failed the transient otoacoustic emission test. The low number of children undergoing these procedures precluded the correlation.
ResultsThroughout this study, 645 (96.3%) of 670 neonates born in the maternity attended to neonatal hearing screening tests. It was not possible to find and/or reschedule the 25 neonates who did not attend and, consequently, there is no information as to their hearing status.
The percentage of “passed” infants in this hearing screening program was 98.5% (635), of “failed” babies, 0.62% (four), and of non-attendance for completion of the diagnostic process, 0.93% (six). Among those babies who passed, 92.6% (588) were discharged and 7.4% (47) were referred for monitoring, due to the presence of some risk factor (Fig. 1).
Infants who failed were referred for diagnostic procedures, and their audiological evaluation results showed the presence of conductive-type hearing loss in all cases (three with bilateral and one with unilateral loss).
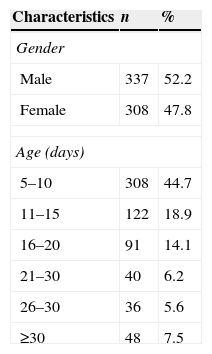
When analyzing the variable “age”, it was observed that 308 (47.75%) infants were submitted to our hearing screening program at 5–10 days of age; the mean time of assessment was 14 days. As to the variable “gender,” the attendance for both genders was similar (Table 1).
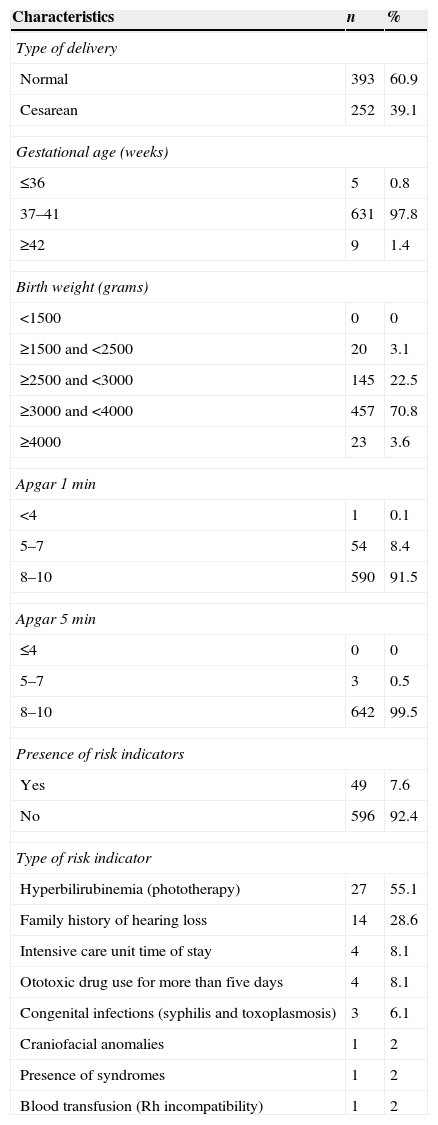
Regarding perinatal characteristics, it was found that most infants had had a normal (60.9%) at term (97.8%) childbirth, with mean weight of 3248g and with appropriate one- and five-minute Apgar scores (Table 2).
Perinatal characteristics of babies screened in the program.
| Characteristics | n | % |
|---|---|---|
| Type of delivery | ||
| Normal | 393 | 60.9 |
| Cesarean | 252 | 39.1 |
| Gestational age (weeks) | ||
| ≤36 | 5 | 0.8 |
| 37–41 | 631 | 97.8 |
| ≥42 | 9 | 1.4 |
| Birth weight (grams) | ||
| <1500 | 0 | 0 |
| ≥1500 and <2500 | 20 | 3.1 |
| ≥2500 and <3000 | 145 | 22.5 |
| ≥3000 and <4000 | 457 | 70.8 |
| ≥4000 | 23 | 3.6 |
| Apgar 1 min | ||
| <4 | 1 | 0.1 |
| 5–7 | 54 | 8.4 |
| 8–10 | 590 | 91.5 |
| Apgar 5min | ||
| ≤4 | 0 | 0 |
| 5–7 | 3 | 0.5 |
| 8–10 | 642 | 99.5 |
| Presence of risk indicators | ||
| Yes | 49 | 7.6 |
| No | 596 | 92.4 |
| Type of risk indicator | ||
| Hyperbilirubinemia (phototherapy) | 27 | 55.1 |
| Family history of hearing loss | 14 | 28.6 |
| Intensive care unit time of stay | 4 | 8.1 |
| Ototoxic drug use for more than five days | 4 | 8.1 |
| Congenital infections (syphilis and toxoplasmosis) | 3 | 6.1 |
| Craniofacial anomalies | 1 | 2 |
| Presence of syndromes | 1 | 2 |
| Blood transfusion (Rh incompatibility) | 1 | 2 |
As for risk indicators for hearing loss, such occurrence was noted in a minority of infants (7.6%), with a mean of risk indicators/baby of 1.12; the most prevalent risk factor was hyperbilirubinemia treated with phototherapy (Table 2).
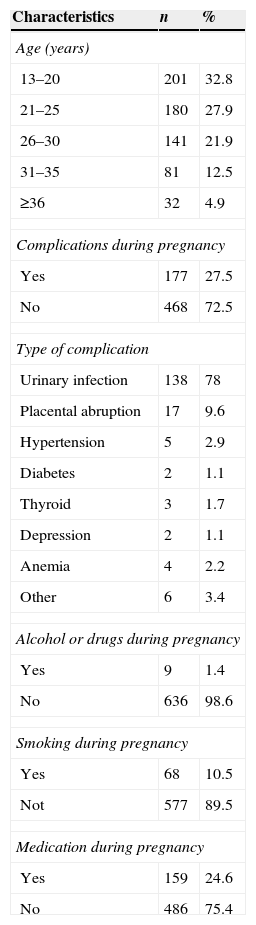
In the mothers’ profile analysis, most were of the age group between 16 and 25 years (57.82%), did not smoke (89.5%), did not use alcohol or drugs (98.6%), and had no complications during pregnancy (72.5%). The most frequent gestational complication was urinary tract infection (Table 3).
Gestational profile of mothers of babies screened in the program.
| Characteristics | n | % |
|---|---|---|
| Age (years) | ||
| 13–20 | 201 | 32.8 |
| 21–25 | 180 | 27.9 |
| 26–30 | 141 | 21.9 |
| 31–35 | 81 | 12.5 |
| ≥36 | 32 | 4.9 |
| Complications during pregnancy | ||
| Yes | 177 | 27.5 |
| No | 468 | 72.5 |
| Type of complication | ||
| Urinary infection | 138 | 78 |
| Placental abruption | 17 | 9.6 |
| Hypertension | 5 | 2.9 |
| Diabetes | 2 | 1.1 |
| Thyroid | 3 | 1.7 |
| Depression | 2 | 1.1 |
| Anemia | 4 | 2.2 |
| Other | 6 | 3.4 |
| Alcohol or drugs during pregnancy | ||
| Yes | 9 | 1.4 |
| No | 636 | 98.6 |
| Smoking during pregnancy | ||
| Yes | 68 | 10.5 |
| Not | 577 | 89.5 |
| Medication during pregnancy | ||
| Yes | 159 | 24.6 |
| No | 486 | 75.4 |
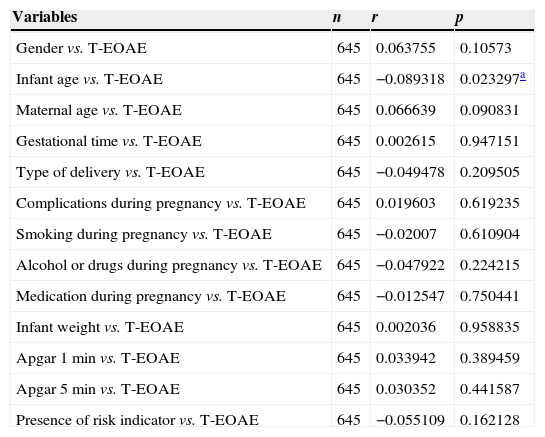
By correlating the variables studied with the results of transient otoacoustic emissions, we observed only a significant negative correlation for infant's age: the higher the infant's age, the lower the “failed” rate (Table 4).
Correlation among variables studied with the results of transient evoked otoacoustic emissions.
| Variables | n | r | p |
|---|---|---|---|
| Gender vs. T-EOAE | 645 | 0.063755 | 0.10573 |
| Infant age vs. T-EOAE | 645 | −0.089318 | 0.023297a |
| Maternal age vs. T-EOAE | 645 | 0.066639 | 0.090831 |
| Gestational time vs. T-EOAE | 645 | 0.002615 | 0.947151 |
| Type of delivery vs. T-EOAE | 645 | −0.049478 | 0.209505 |
| Complications during pregnancy vs. T-EOAE | 645 | 0.019603 | 0.619235 |
| Smoking during pregnancy vs. T-EOAE | 645 | −0.02007 | 0.610904 |
| Alcohol or drugs during pregnancy vs. T-EOAE | 645 | −0.047922 | 0.224215 |
| Medication during pregnancy vs. T-EOAE | 645 | −0.012547 | 0.750441 |
| Infant weight vs. T-EOAE | 645 | 0.002036 | 0.958835 |
| Apgar 1min vs. T-EOAE | 645 | 0.033942 | 0.389459 |
| Apgar 5min vs. T-EOAE | 645 | 0.030352 | 0.441587 |
| Presence of risk indicator vs. T-EOAE | 645 | −0.055109 | 0.162128 |
T-EOAE, transient-evoked otoacoustic emissions; p, significance level; r, correlation coefficient.
The implementation of universal newborn hearing screening programs intends to minimize and/or prevent hearing impairment related deficits in language, social, emotional, and cognitive development of children, regardless of the presence of risk indicators.
In the literature reports it can be seen that, for a screening program to be considered universal, at least 95% of neonates must be evaluated.1 Based on this index, it can be said that this program was universal, since it covered 96.3% of newborns in this maternity. Thus, the importance of universal screening in this maternity is reinforced by the fact that the majority of the population cared for is considered as at low risk for hearing loss.
A study conducted in South Africa showed that the prevalence of sensorineural hearing loss in newborns is approximately 1/3 in 1000 children with low risk for hearing loss.29 In Brazil, studies report a prevalence of approximately 0.9% for hearing impairment, regardless of the presence of risk indicators,30,31 and that 50% of hearing loss cases are identified in children considered at low risk.1,32,33
Another fact observed in this study, related to its scope, is the low dropout rate, which corroborates the findings described in the literature.18,34 However, other studies have reported a high dropout rate as a major challenge for newborn hearing screening programs.35,36
The fact that the hearing screening tests were performed on the babies on the day scheduled for puerperal consultation and the active search system conducted by the health workers of the Basic Health Units of the municipality are possible explanations for this low dropout rate.
The implementation of newborn hearing screening programs in this country, especially in maternities that serve people with a lower socioeconomic status, is confronted with many difficulties that hinder its efficiency, since the dropout rate of this population during the process of newborn hearing screening is very high. Included among the reasons for not attending to the recommended returns are a lack of information of parents about the causes and symptoms, and the impact of hearing loss on the overall development of the child, a prevalent idea among mothers that their children have no risk of suffering a hearing loss, and anxiety triggered by the knowing that their children are being tested.36
By analyzing another indicator of effectiveness of the program, it was observed that the age group with highest concentration of screening tests performed was that of infants between the fifth and tenth day of life (mean, 12); this finding is consistent with the literature, which calls for screening tests in the first month of life.1
The percentage of hearing loss found in our population was 0.62%. In the literature, lower and similar rates were found, ranging between 0.1% and 0.5%;18,22,36–38 but higher rates were also found, ranging between 1.8% and 3.44%.39–41 This variation may be due to the difference among populations studied and also among methodologies employed.
The percentage of children who failed and thus were referred for diagnosis was 1.7% – less than those 4% reported in the literature.1,42 By analyzing the use of transient otoacoustic emissions as an initial test, it was found that 95% of infants passed the exam. In the Brazilian literature, this percentage ranged from 85% to 96.78%; some of these studies corroborate this finding, while others do not confirm it.19,40,43,44
The findings of this study, including the effectiveness of the program and lack of sensorineural hearing loss, are justified by the fact that most of the evaluated babies showed no risk indicators for hearing loss, by the number of babies evaluated in the period and, finally, because the screening test was held in the same day of puerperal consultation, allowing the realization of several procedures on the same day and place.
Another aspect addressed by the authors was the characterization of mothers and babies that attended this program. It must be borne in mind that 32.8% of mothers in this study were aged 13–19 years, thus being classified as teenagers according to the World Health Organization.45
One study reported that occurrence of pregnancy in this period is a consistent public health problem, because of the greater risk for the mother and her infant, in addition to its strong biological, psychological, and social impact. The risk of maternal death for women aged 15–19 years is twice the risk for women aged 20–24 years.46 However, in this study there were few complications in this population.
Finally, by correlating these variables with the results of transient otoacoustic emissions, a significant negative correlation was noted only for the infant's age: the higher the age, the lower the failure rate. However, there was no correlation among other variables studied, including presence of risk indicators, with the results of the examination.
One study indicates that failure rates can vary from 5% to 20% when the screening procedure is performed with otoacoustic emissions during the first 24h, falling to 3% when the test is held between 24 and 48h after birth.42
In Brazil, most services perform their neonatal hearing screening before discharging the infant; however, there is no rule indicating whether the test should be conducted during the first 24h of life or at a later time, during the first 48h of life. Thus, it remains unclear if the infant's life span affects the outcome of neonatal hearing screening tests.47
With respect to the presence of risk factors for hearing loss, the literature confirms that there is no correlation between risk factors for hearing loss and the result of neonatal hearing screening procedures;26 however, it must be said that other studies found correlation between these variables.37 The most common risk factor in this population was hyperbilirubinemia treated with phototherapy; this finding agrees with data in the literature.27 This factor can compromise the newborn hearing ability, with inner ear and central auditory pathway injury.48
ConclusionThe universal newborn hearing screening program implemented in this maternity ward was effective and meets national and international recommendations. As for the participants’ profile, the population consisted of young mothers with few alterations in their pre-, peri-, and post-natal periods, and with healthy infants. The only variable that influenced the results of transient otoacoustic emissions after hospital discharge was the age at which the infants were evaluated.
Funding sourceThis study was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Kemp AAT, Delecrode CR, da Silva GC, Martins F, Frizzo ACF, Cardoso ACV. Neonatal hearing screening in a low-risk maternity hospital in São Paulo state. Braz J Otorhinolaryngol. 2015;81:505–13.
Institution: Universidade Estadual Paulista “Júlio de Mesquita Filho”, School of Philosophy and Sciences, Marília Campus, SP, Brazil.