Cochlear implants have revolutionized the way patients affected by severe hearing loss experience the world. Neurelec developed a fixation system with two titanium screws that requires no skull bone drilling.
ObjectiveTo describe the outcomes and procedure-related details of a series of patients implanted with the Digisonic® SP cochlear implant.
MethodThis retrospective study analyzed patients submitted to cochlear implant placement within a period of 18 months. All patients had postlingual hearing impairment. Data was collected from patient charts and standard questionnaires answered by the surgeons in charge of carrying out the procedures.
ResultsThe six patients offered the Digisonic® SP cochlear implants were operated by experienced surgeons. The procedures took 95 to 203 minutes (mean = 135') to be completed, which is less time than what has been described for other fixation approaches. No complications were recorded and hearing improvement was satisfactory.
ConclusionThe Digisonic® SP cochlear implant developed by Neurelec offered good audiological results for adult patients, shorter surgery time, and no surgical or postoperative complications.
Cochlear implants have revolutionized the way patients affected by severe hearing loss experience the world. Good outcomes with improved speech perception in patients of all age ranges have been observed1-3. Cochlear implants improve patient quality-of-life even more significantly in subjects aged 65 years and older4.
The implantation procedure is safe and reliable, but complications occur in about 16% of the patients. The most frequent adverse event relates to the insertion of electrodes into the cochlea, seen in about 4% of the cases5.
Migration of internal components has been described by many authors and may lead to cochlear implant malfunction and local infection6-8. Various implantation procedures have been described in the literature, with less invasive approaches gaining significant attention9-11.
The production of the bed on which the internal component is positioned is an important and onerous stage in the procedure, and has been the target of significant investment by cochlear implant makers12,13. More is thus spent in the development of surgical instruments and new materials such as titanium plates, propylene mesh, GORE-TEX, absorbable materials, and others14-16.
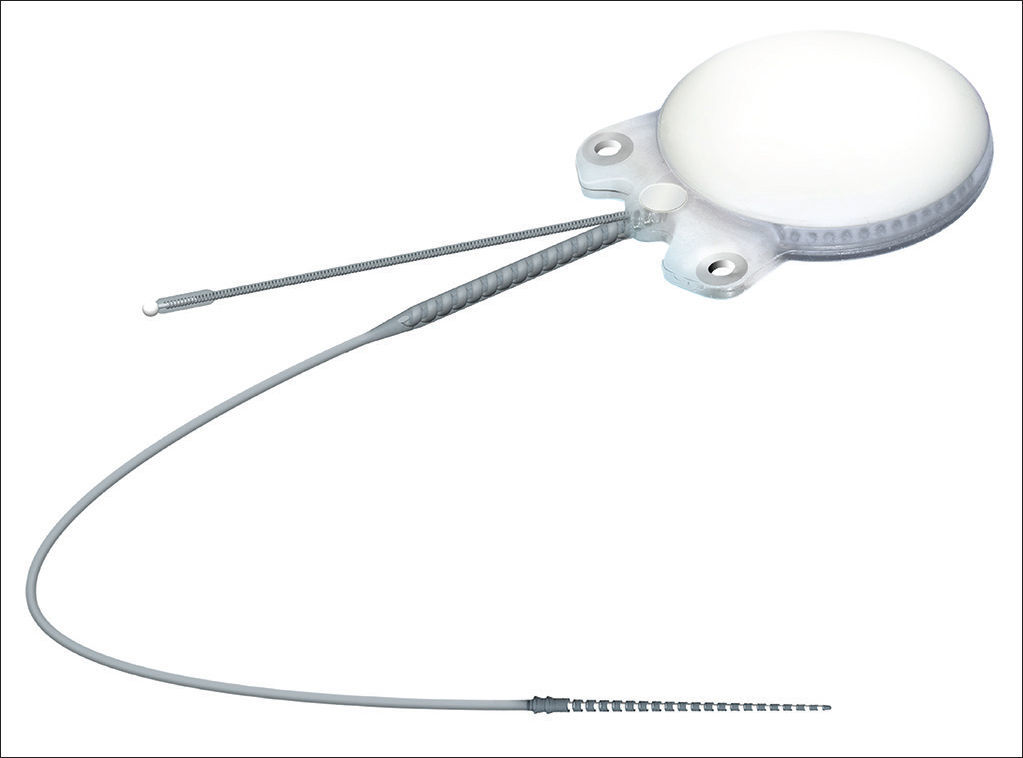
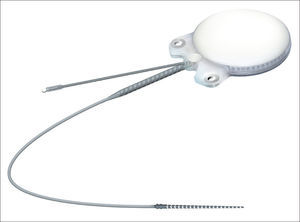
In regards to internal component fixation, Neurelec Inc. (Sophia-Antipolis, France) developed a fixation system with two titanium screws that requires no skull bone drilling17 (Figure 1).
Therefore, this study aimed to describe the outcomes and procedure-related details of a series of patients implanted with the Digisonic® SP cochlear implant and discuss the postoperative clinical and audiological findings observed in the last 18 months.
METHODThis retrospective study was carried out in a specialized tertiary care hospital. Patients implanted with Digisonic® SP cochlear devices were assessed for a period of 18 months.
A digital data collection protocol was designed. The following parameters were analyzed: age, gender, hearing loss etiology, time with hypacusis, side of implantation, pre and postoperative audiometric data (audiometry and speech perception testing), length of surgery, time to fixate the internal component, surgery complications, follow-up time in months. All patients in this series had post-lingual hearing loss (hearing was lost after they had developed speech and language skills).
Data was collected from patient charts, and a standard questionnaire answered by the surgeons who carried out the procedures.
The identity of the patients was not disclosed, as required by the ethical principles of the institution in which the study was conducted.
The patientsThe patients selected for the study had Digisonic® SP cochlear devices implanted within a period of 18 months. All subjects followed a preoperative protocol in which the etiology of the impairment was investigated through lab workup, genetic tests, CT and MRI scans of the ears and mastoids, psychological evaluation, and thorough speech and hearing assessment.
The deviceThe Digisonic® SP cochlear implant system - made up by cochlear implant Digisonic® SP and speech processors Digi SP or Digi SP'K - was launched by French company Neurelec S.A. in 2004. This device belongs to the latest generation of implantable components developed by Neurelec and offers several advancements in relation to previous generation devices. The device's main features include an increased number of electrodes in the beam to allow for a greater number of active channels for stimulation and better spectral representation inside the cochlea, and a fixation system for the receiver-stimulator that uses two titanium screws and raises the stimulation rate through the “Mean Peak Interleaved Sampling” (MPIS) sound processing strategy.
Device internal componentInternal component Digisonic® SP developed by Neurelec is shown in Figure 1.
The receiver-stimulator (RS) is made up of a convex ceramic capsule, a titanium base plate, both coated with biocompatible silicone. Digisonic® SP is extremely compact, and both its electronic components used in signal decoding and its internal magnet are comprehended by one single structure with a diameter of 30 mm called the monoblock.
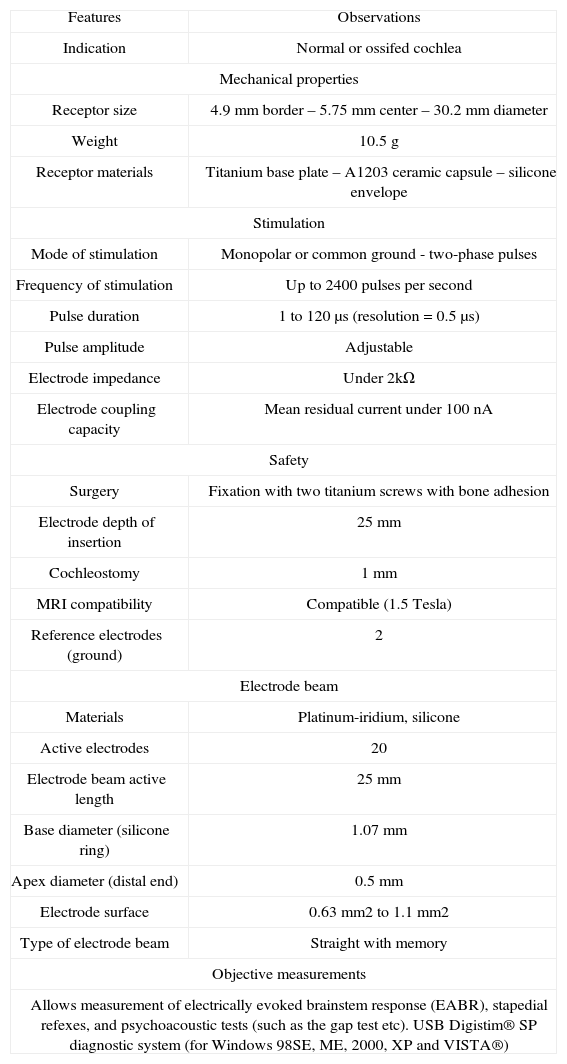
Table 1 describes the characteristics of Digisonic® SP.
Digisonic® SP technical features.
| Features | Observations |
| Indication | Normal or ossifed cochlea |
| Mechanical properties | |
| Receptor size | 4.9 mm border – 5.75 mm center – 30.2 mm diameter |
| Weight | 10.5 g |
| Receptor materials | Titanium base plate – A1203 ceramic capsule – silicone envelope |
| Stimulation | |
| Mode of stimulation | Monopolar or common ground - two-phase pulses |
| Frequency of stimulation | Up to 2400 pulses per second |
| Pulse duration | 1 to 120 μs (resolution = 0.5 μs) |
| Pulse amplitude | Adjustable |
| Electrode impedance | Under 2kΩ |
| Electrode coupling capacity | Mean residual current under 100 nA |
| Safety | |
| Surgery | Fixation with two titanium screws with bone adhesion |
| Electrode depth of insertion | 25 mm |
| Cochleostomy | 1 mm |
| MRI compatibility | Compatible (1.5 Tesla) |
| Reference electrodes (ground) | 2 |
| Electrode beam | |
| Materials | Platinum-iridium, silicone |
| Active electrodes | 20 |
| Electrode beam active length | 25 mm |
| Base diameter (silicone ring) | 1.07 mm |
| Apex diameter (distal end) | 0.5 mm |
| Electrode surface | 0.63 mm2 to 1.1 mm2 |
| Type of electrode beam | Straight with memory |
| Objective measurements | |
| Allows measurement of electrically evoked brainstem response (EABR), stapedial refexes, and psychoacoustic tests (such as the gap test etc). USB Digistim® SP diagnostic system (for Windows 98SE, ME, 2000, XP and VISTA®) | |
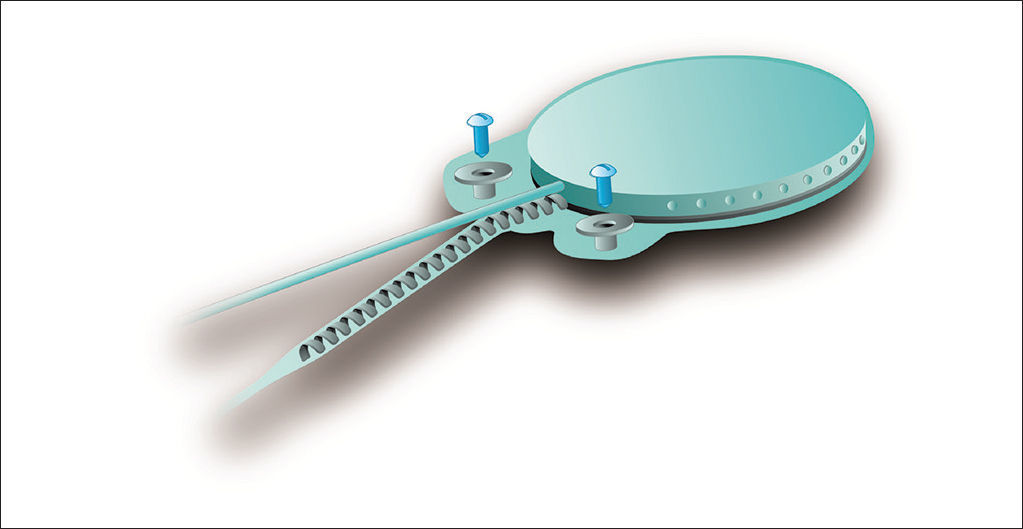
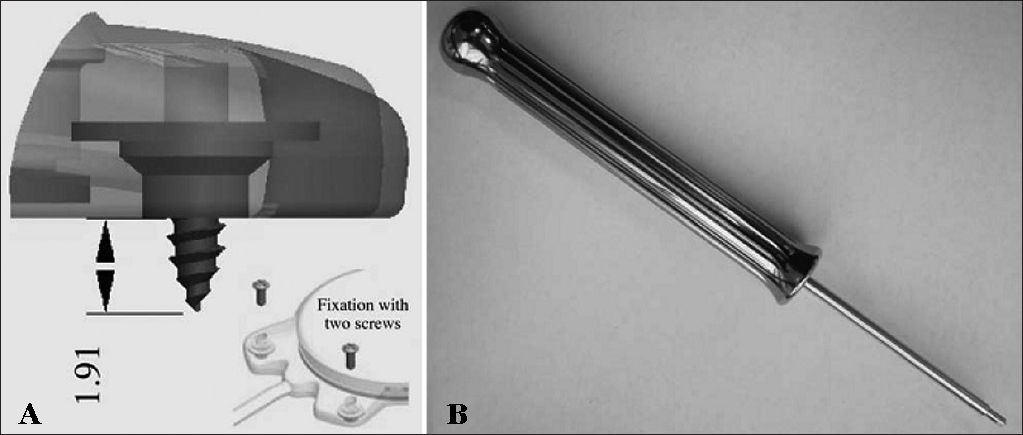
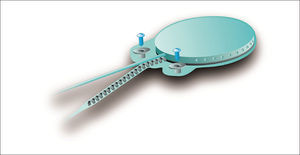
The device's fixation is done by two 3.4 mm titanium screws bolted into two small silicone-coated titanium orifices with 5 mm in diameter positioned in the ends of the receiver-stimulator, as shown in Figures 2 and 3. The screws are driven 1.91 mm into bone tissue17.
The compact structure and the fixation screws of the Digisonic® SP allow for quicker, less invasive surgery without the need to drill or suture bone tissue to position or fixate the implant17.
Digisonic® SP has atraumatic flexible screws that adjust quickly to the site in which they were positioned and connect firmly to the RS. The beam is made of 20 platinum-iridium electrodes, which allow the stimulation of up to 20 channels along the cochlea, with an active length of 25 mm and a stimulation rate of up to 1,000 pulses per second for each stimulation channel enabled by processing strategy MPIS. It is also equipped with silicone rings to facilitate insertion18.
The internal device contains a two-way telemetry system to record electrode impedance. Electrode impedance is recorded through diagnostic interface Digistim SP and software program Digistim for Windows SP® version 1.9.15, in which it is also possible to perform other objective measurements such as electrically evoked auditory brainstem responses (EABR) and electrically evoked stapedius reflex thresholds (ESRT)18.
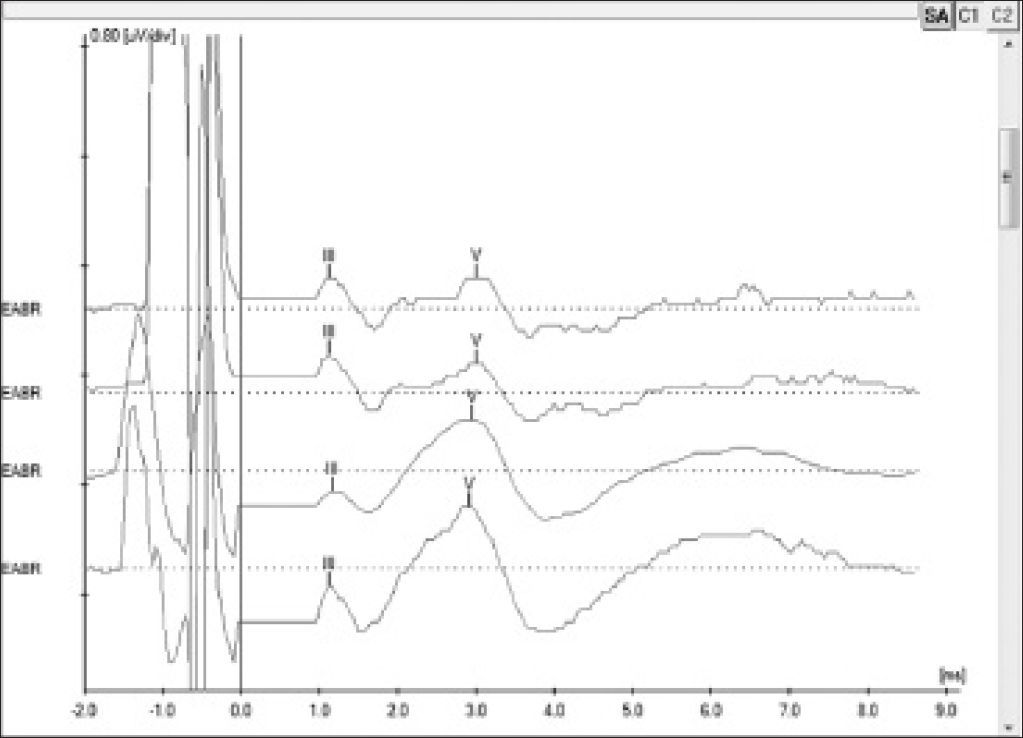
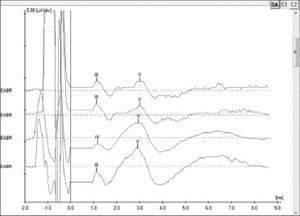
EABR measurement is carried out routinely during surgery to verify device function and the stimulation of peripheral auditory nerve fibers19. In addition to that, EABR can also be used to predict the psychophysical levels to program the speech processor20, a particularly important step in the treatment of pediatric patients. EABR is measured at the end of surgery with the patient still under general anesthesia.
The procedure comprehends the electrical stimulation of nerve fibers through the electrodes inserted in the cochlea and the acquisition of responses through a conventional BAEP measurement device and a synchronization cord. It is possible to view waves II, III, and V, the last two being the most commonly observed; wave V is indicative of effective cochlear nerve stimulation. The absolute latencies of these waves are reduced when compared to conventional BAEP, given that the stimulation on EABR is performed directly in the cochlear nerve through the electrodes in the implant beam20 (Figure 4).
Patients using the Digisonic® SP implant can undergo magnetic resonance imaging in scanners of up to 1.5 Tesla, if recommendations are followed18.
RESULTSTable 1 shows the characteristics and technical details of the device used in the described procedures.
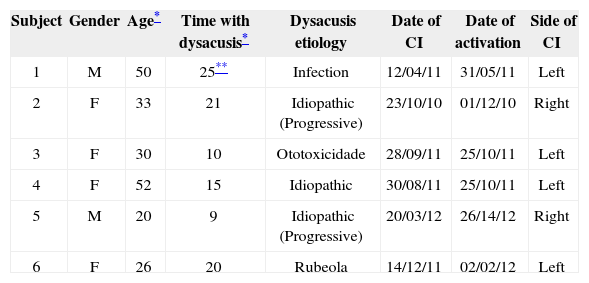
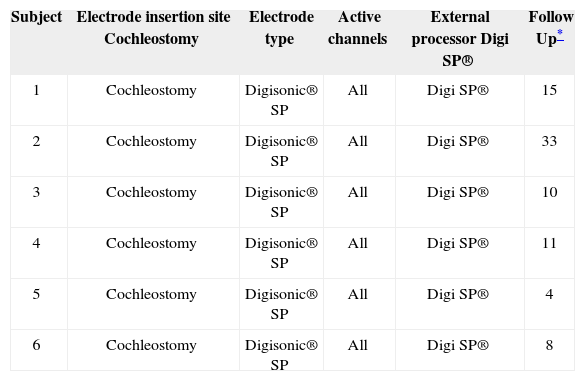
Tables 2 and 3 show general and specific data of the patients implanted with Digisonic® SP.
General implant patient data.
| Subject | Gender | Age* | Time with dysacusis* | Dysacusis etiology | Date of CI | Date of activation | Side of CI |
|---|---|---|---|---|---|---|---|
| 1 | M | 50 | 25** | Infection | 12/04/11 | 31/05/11 | Left |
| 2 | F | 33 | 21 | Idiopathic (Progressive) | 23/10/10 | 01/12/10 | Right |
| 3 | F | 30 | 10 | Ototoxicidade | 28/09/11 | 25/10/11 | Left |
| 4 | F | 52 | 15 | Idiopathic | 30/08/11 | 25/10/11 | Left |
| 5 | M | 20 | 9 | Idiopathic (Progressive) | 20/03/12 | 26/14/12 | Right |
| 6 | F | 26 | 20 | Rubeola | 14/12/11 | 02/02/12 | Left |
Specific implant patient data.
| Subject | Electrode insertion site Cochleostomy | Electrode type | Active channels | External processor Digi SP® | Follow Up* |
|---|---|---|---|---|---|
| 1 | Cochleostomy | Digisonic® SP | All | Digi SP® | 15 |
| 2 | Cochleostomy | Digisonic® SP | All | Digi SP® | 33 |
| 3 | Cochleostomy | Digisonic® SP | All | Digi SP® | 10 |
| 4 | Cochleostomy | Digisonic® SP | All | Digi SP® | 11 |
| 5 | Cochleostomy | Digisonic® SP | All | Digi SP® | 4 |
| 6 | Cochleostomy | Digisonic® SP | All | Digi SP® | 8 |
Table 4 shows the data on surgery-related occurrences and length of the procedure.
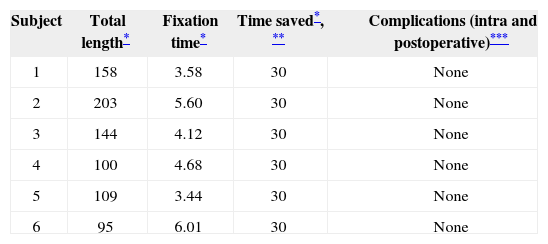
Surgery implant patient data.
| Subject | Total length* | Fixation time* | Time saved*, ** | Complications (intra and postoperative)*** |
|---|---|---|---|---|
| 1 | 158 | 3.58 | 30 | None |
| 2 | 203 | 5.60 | 30 | None |
| 3 | 144 | 4.12 | 30 | None |
| 4 | 100 | 4.68 | 30 | None |
| 5 | 109 | 3.44 | 30 | None |
| 6 | 95 | 6.01 | 30 | None |
time to fixate internal component as reported by surgeon. The mean time to the completion of this portion of the procedure (production of internal component niche) was calculated by the analysis of 10 random cases done within the same time period using different fixation modes (temporal bone drilling to make the niche);
MRI and CT scans did not reveal radiological alterations.
EABR and ERST measurements were performed in all procedures at the end of surgery with the patients still under general anesthesia. Measurement outcomes were satisfactory and showed the device was functioning properly.
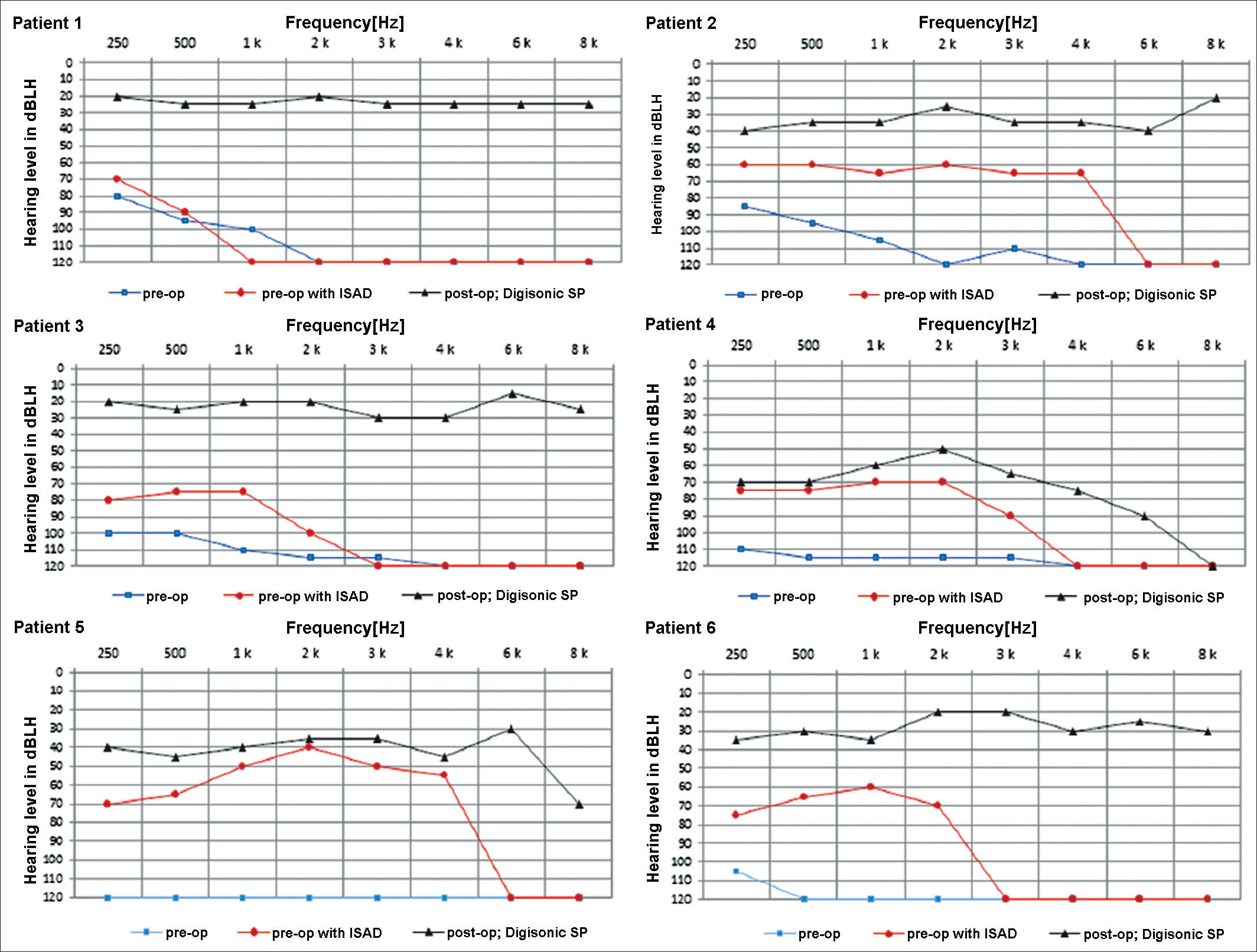
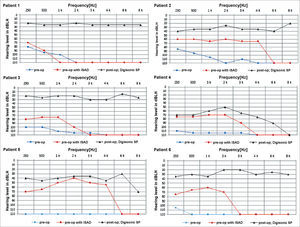
The graphs below show the pre and postoperative audiometric test results (Figure 5).
DISCUSSIONThe Digisonic® SP cochlear implant developed by Neurelec has a fixation system with two titanium screws, which does not require the production of a niche to place the cochlear implant internal component or any drilling on the patient's skull bone. In addition to reducing the risks and complications associated with the production of the cochlear implant niche, this fixation system reduces the length of surgery.
In our study, six patients were implanted with Digisonic® SP by experienced surgeons, and surgery length ranged from 95 and 203 minutes, with a mean length of 135 minutes. The mean length of a conventional cochlear device implantation procedure is 255 minutes in the hands of experienced surgeons using the S-shaped retroauricular incision to make the implant niche, and 200 minutes when using a small retroauricular incision and a subperiosteal pouch21. Therefore, length of surgery in our series was shorter than that of procedures using other modes of fixation.
Studies looking into the cost of cochlear implants use a wide array of methods, and cost estimates may vary significantly depending on what is considered as part of the cost22. However, according to the literature, the cost of a unilateral implant in a patient with post-lingual hearing loss ranges between € 30,026 (USD 21,018) and € 45,770 (USD 32,039), with the device accounting for most of the cost23. In Brazil, the cost of a Digisonic® SP is similar to the cost of a conventional fixation device.
Despite the high cost, the benefits of cochlear implants far outweigh the costs as they enable hearing rehabilitation, improved communication, and better quality-of-life for deaf patients, with even more evident results seen in younger patients23-25.
The patients in our series had no complications, and the level of auditory gain was satisfactory in all cases. In general terms, mean complication rates of 16% have been described in cochlear device implantation procedures5. Despite the small size of our sample, our results were better than average.
CONCLUSIONThe Digisonic® SP cochlear implant developed by Neurelec presented good audiological outcomes in our series, shorter length of surgery, and no intra or postoperative complications.
Paper submitted to the BJORL-SGP (Publishing Management System – Brazilian Journal of Otorhinolaryngology) on August 6, 2012; and accepted on October 2, 2012. cod. 9938.