Coordination
Wilma T. Anselmo-Lima e Eulalia Sakano
Participants
André Alencar, Atílio Fernandes, Edwin Tamashiro, Elizabeth Araújo, Érica Ortiz, Fabiana Cardoso Pereira Valera, Fábio Pinna, Fabrizio Romano, Francini Padua, João Mello Jr., João Teles Jr., José E. L. Dolci, Leonardo Balsalobre, Macoto Kosugi, Marcelo H. Sampaio, Márcio Nakanishi, Marco César, Nilvano Andrade, Olavo Mion, Otávio Piltcher, Reginaldo Fujita, Renato Roithmann, Richard Voegels, Roberto E. Guimarães, Roberto Meireles, Shirley Pignatari, Victor Nakajima
For the purpose of citation
Wilma Terezinha Anselmo Lima, Eulalia Sakano, Edwin Tamashiro, Elizabeth Araújo, Érica Ortiz, Fábio Pinna, Fabrizio Romano, Francini Padua, João Mello Jr., João Teles Jr., José E. L. Dolci, Leonardo Balsalobre, Macoto Kosugi, Marcelo H. Sampaio, Márcio Nakanishi, Marco César, Nilvano Andrade, Olavo Mion, Otávio Piltcher, Reginaldo Fujita, Renato Roithmann, Richard Voegels, Roberto E. Guimarães, Roberto Meireles, Victor Nakajima, Fabiana Cardoso Pereira Valera, Shirley Pignatari
IntroductionRhinosinusitis (RS) is an inflammatory process of the nasal mucosa, and according to the evolution of signs and symptoms, it is classified as acute (ARS; < 12 weeks) or chronic (CRS; ≥ 12 weeks). According to the severity of the condition, it is classified as mild, moderate, or severe. Disease severity is graded using a visual analog scale (VAS) (Fig. 1), from 0 to 10cm. Patients are asked to quantify, from 0-10 at the VAS, the degree of discomfort caused by their symptoms, with 0 meaning no discomfort and 10 the highest discomfort. Severity is then classified as: mild; 0-3cm; moderate; > 3-7cm; and severe; > 7-10cm.1
Although VAS has only been validated for CRS in adults, the European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS) 20121 also recommends its use in ARS. There are several specific questionnaires for rhinosinusitis, but in practice, most have limited application, particularly in acute cases.2–4
Acute rhinosinusitisDefinitionARS is an inflammatory process of the nasal mucosa of sudden onset, lasting up to 12 weeks. It may occur one or more times in a given period of time, but always with complete remission of signs and symptoms between episodes.
ClassificationThere are several classifications for RS. One of the most often used is the etiological classification, which is based mainly on symptom duration:1
- •
Common cold or viral ARS: a condition that is usually self-limited, in which symptoms last less than ten days;
- •
Post-viral ARS: defined when there is symptom worsening after five days of disease, or when symptoms persist for more than ten days;
- •
Acute bacterial RS (ABRS): a small percentage of patients with post-viral ARS can develop ABRS.
Viral ARS or common cold symptoms traditionally last less than ten days. Symptom worsening around the fifth day, or persistence beyond ten days (and less than 12 weeks), can represent a case of post-viral RS. It is estimated that a small percentage of post-acute viral RS (around 0.5% to 2% of cases) develop into a bacterial infection.
Regardless of duration, the presence of at least three of the signs/symptoms below may suggest ABRS:
- •
Nasal discharge (with unilateral predominance) and purulent secretion in the nasopharynx;
- •
Local intense pain (with unilateral predominance);
- •
Fever > 38°C;
- •
Elevated erythrocyte sedimentation rate or C-reactive protein levels;
- •
“Double worsening”: acute relapse or deterioration after the initial stage of mild symptoms.
Exposure to increasing levels of humidity, but not fungi, has been associated with ARS.5 Seasonal variations have also been reported in the literature, with increased incidence of ARS during the winter months.5–9 Exposure to air pollution,10–12 irritants used in the production of pharmaceuticals,13 in photocopiers,14 and smoke from forest fires,15 have all been associated with increased prevalence of ARS symptoms.
Anatomical factorsAnatomical variations including Haller cells, concha bullosa, nasal septal deviation, choanal atresia, pharyngeal tonsil hypertrophy, nasal polyps, hypoplastic sinuses, and odontogenic origin of infections may be associated with ARS.10,16–18
AllergyThe role of allergy in ARS is controversial. There have been studies that assessed the association between allergic rhinitis and ARS,19–35 while others dismissed such an association.35–37
Ciliary injuryCiliary injury has been considered a characteristic of viral and bacterial RS.38 It includes the loss of cilia and ciliated cells, as well as alteration of the normal mucociliary transport. However, smoking and allergies have also been implicated in the alteration of the mucociliary transport,39,40 and the alteration in the mucociliary clearance in patients with allergic rhinitis has been shown to predispose to ARS.22
Primary ciliary dyskinesia (PCD)This is a rare autosomal recessive disease, in which the cilia are either immotile or beat with a pattern incompatible with mucus transport in the airway. PCD is associated with chronic upper airway symptoms such as rhinorrhea, episodic facial pain, anosmia, and bronchiectasis.41 Newborns may present rhinorrhea from the first day of life.42,43 There are no data on the frequency of ARS episodes in this group of patients. According to the European Respiratory Society Task Force on Primary Ciliary Dyskinesia, recurring ARS is rare in patients with PCD, although the episodes should be treated with appropriate antibiotics and for a prolonged period of time.44,45
SmokingChildren living in environments with adult smokers are more prone to episodes of ARS than those who are not exposed to this environment.46 Active smokers with ongoing allergic inflammation have increased susceptibility to ARS when compared to non-smokers during the course of allergic inflammation, suggesting that exposure to cigarette smoke and allergic inflammation are mediated by different pathways and possible synergistic mechanisms.47
Smoking (active and passive) has been shown to alter the normal bacterial flora present in the nasopharynx, resulting in greater potential for colonization of pathogens than in nonsmokers.48 Once smoking is discontinued, the microbial population begins to show the same pattern found in nonsmokers.49
Gastroesophageal refluxLittle is known about the association between ARS and gastroesophageal reflux. Although studies conducted between 1997 and 2006 have observed a significant association between the two diseases,50 a recent systematic review found a weak association between acid reflux, nasal symptoms, and ARS.51
Anxiety and depressionStates of impaired mental health, anxiety, or depression are often associated with increased susceptibility to ARS.52 However, the involved mechanisms remain unclear.
Antimicrobial resistanceThe main pathogens of ABRS include S. pneumoniae, H. influenzae, S. pyogenes, M. catarrhalis, and S. aureus.38 Despite the problems related to bacterial resistance, it is estimated that approximately 80% of cases of mild ARS respond to amoxicillin at a dose of 70 to 90mg/kg/day. A study by Principi and Esposito53 demonstrated that most cases of ARS caused by H. influenzae and M. catarrhalis and approximately 15% of those caused by S. pneumoniae resolve spontaneously. Lin et al. observed that 70% ofpneumoniae and 71.4% of H. influenzae cases isolated from 69 children were resistant to amoxicillin and clavulanate.19
Concomitant chronic diseaseConcomitant chronic disease (bronchitis, asthma, cardiovascular disease, diabetes mellitus, or malignant tumor) in children has been associated with an increased incidence of ARS after influenza.54
Clinical diagnosisSigns and symptomsAt primary health care levels and for epidemiological purposes, ARS can be diagnosed based on symptoms alone, without detailed otorhinolaryngological assessment and/or without imaging studies.
In these cases, the distinction between the types of ARS is mainly determined through clinical history and physical examination performed by general practitioners and specialists, whether or not otorhinolaryngologists. It is worth mentioning that, at the time of the examination, patients may not report symptom worsening if not asked carefully. The report of symptoms occurring a few days before with a recurrence of symptoms just before evaluation is frequent. Health care professional should realize that, in most cases, this may represent the evolution of the same disease, from a viral to a post-viral ARS, rather than two distinct infections. Subjective evaluation of patients with ARS and their diagnosis is based on the presence of two or more of the following cardinal symptoms:1
- •
Nasal obstruction/congestion;
- •
Anterior or posterior nasal discharge/rhinorrhea (most often, but not necessarily, purulent);
- •
Facial pain/pressure/headache;
- •
Disorder of olfaction.
In addition to the symptoms described above, odynophagia, dysphonia, cough, and ear fullness and pressure, as well as systemic symptoms such as asthenia, malaise, and fever, may also occur. The few studies on the frequency of these symptoms in ARS in the community have shown great variability.55–57 The possibility of ABRS is greater in the presence of three or more of the following signs and symptoms:1
- •
Nasal secretion/presence of pus in the nasal cavity with unilateral predominance;
- •
Local pain with unilateral predominance;
- •
Fever > 38°C;
- •
Deterioration/worsening of symptoms after the initial period of the disease;
- •
Elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels.
ARS symptoms have a characteristically abrupt onset, without a recent history of RS symptoms. In the acute exacerbation of CRS, the diagnostic criteria and treatments similar to those used for ARS should be used.1 “Cough”, although considered an important symptom according to most international guidelines, is not one of the cardinal symptoms in this document. Nonetheless, in the pediatric population, cough is considered one of the four cardinal symptoms, replacing olfaction disorders.1,58 Gwaltney et al.,59 when studying the symptoms of spontaneous rhinosinusal infections by rhinovirus in relation to the time of onset and duration, observed that the peak of typical symptoms such as nasal obstruction, rhinorrhea, and cough occurs between the second and third days of infection (Fig. 2), with a tendency to decrease thereafter. Symptoms can, however, last for 14 days or more.
Rhinosinusitis symptoms of acute infection caused by rhinovirus in relation to the start time and duration. (Adapted from Gwaltney et al. [1967]).59
Nasal obstruction is one of the important symptoms of ARS and should be assessed together with other patient complaints. In spite of the scarcity with which methods of objective evaluation of nasal obstruction (such as rhinomanometry, nasal peak inspiratory flow, and acoustic rhinometry) are applied in daily practice in patients with ARS, studies have shown a good correlation between the symptoms reported by patients and the objective measurements obtained by these methods.1
Purulent rhinorrhea is often interpreted in clinical practice as indicative of bacterial infection and need for antibiotic use.60,61 However, evidence of this association is limited. Although it is a symptom that appears to increase the chances of positive bacterial culture, in isolation it does not characterize ABRS.62 Purulent rhinorrhea with unilateral predominance and pus in the nasal cavity have a positive predictive value of only 50% and 17%, respectively, for positive bacterial culture obtained by maxillary sinus aspirate.63 Thus, the presence of purulent rhinorrhea does not necessarily indicate the existence of a bacterial infection and should not be used as an isolated criterion for the prescription of an antibiotic.62–64 Decreased olfaction is one of the most difficult symptoms to quantify in clinical practice and usually only is evaluated subjectively. Complaints of hyposmia and anosmia are commonly associated with ARS, and can be assessed with good correlation by employing validated objective tests with subjective scales.65,66 It is important that these tests of olfactory function are translated and culturally and socioeconomically adapted for their use in different populations.67
Facial pain and pressure commonly occur in ARS. When unilateral, facial or dental pain has been considered a predictor of acute maxillary sinusitis.55,68 The complaint of dental pain in the upper teeth abutting on the maxillary sinus showed a statistically significant association with the presence of positive bacterial culture obtained from sinus aspirates, with a predominance of S. pneumoniae and H. influenza.69 However, in another study, the predictive positive value of unilateral facial pain for bacterial infection was only 41%.68
Several studies and guidelines have sought to define the combination of symptoms that best determine the highest probability of bacterial infection and antibiotic response.1 In the study by Carenfelt and Berg,68 the presence of two or more findings (purulent rhinorrhea and unilaterally predominant local pain, pus in the nasal cavity, and bilateral purulent rhinorrhea) showed 95% sensitivity and 77% specificity for the diagnosis of ABRS.
The clinical examination of a patient with ARS should initially comprise assessment of vital signs and physical examination of the head and neck, with special attention aimed at the presence of localized or diffuse facial edema. At oroscopy, posterior purulent oropharyngeal secretions58 are important. Anterior rhinoscopy is a part of the physical examination that should be performed in the primary assessment of patients with rhinosinusal symptoms; although it provides limited information, it may reveal important aspects of the nasal mucosa and secretions.1
Fever may be present in some patients with ARS in the first days of infection59 and, when higher than 38°C, it is regarded as indicative of more severe disease and may indicate the need for more aggressive treatment, especially when associated with other severe symptoms. Fever is also significantly associated with positive bacterial culture obtained from nasal aspirate, especially S. pneumoniae and influenzae.
In patients with ARS, the presence of edema and pain on palpation of the maxillofacial region may be indicative of more severe disease requiring antibiotics, despite the limited data available in literature.60
At the primary health care level, nasal endoscopy is usually not routinely available and is not considered a mandatory examination for ARS diagnosis. When available, it allows the specialist to better visualize the nasal anatomy and to obtain a topographic diagnosis and material for microbiological analysis.1
At the assessment and clinical examination of patients, possible variations between geographical regions and different populations should be considered. Among other factors, climatic, social, economic, and cultural differences, as well as opportunity of access to health care, can change the subjective perception of the disease and potentially generate peculiar clinical features. The importance of this variability is unknown; more studies are needed to establish this.
Complementary examinationsNasal endoscopyAs previously mentioned, it is not a mandatory examination for the diagnosis of ARS, but it may be useful for the assessment of the nasal anatomy, biopsy, and culture. Several microbiological studies have shown a reasonable correlation between the findings collected by puncture from the middle meatus, allowing for a microbiological confirmation of the agent and its therapeutic response. Some authors recommend diagnostic confirmation through nasal endoscopy and culture, as many patients with clinical or radiological evidence of ABRS do not have a positive culture.1,70
C-reactive protein (CRP)Low or normal levels of this protein can identify patients with low likelihood of bacterial infection, preventing unnecessary antibiotic use. Treatment guided by polymerase chain reaction (PCR) has been associated with a reduction in antibiotic use, without affecting the outcome. Although more studies are still required to include this routine diagnostic examination for ABRS, some studies have shown that CRP levels are strongly associated with the presence of changes in computed tomography (CT), and that high CRP levels can be considered predictive of positive bacterial culture from puncture or sinus lavage.69,71,72
Erythrocyte sedimentation rate (ESR)Inflammatory markers such as ESR and plasma viscosity are elevated in ARS, and may reflect disease severity and the need for more aggressive treatment. Their levels are associated with the presence of CT alterations in ARS and values greater than 10 are considered predictive of fluid level or opacity at CT. High values are also predictive of positive bacterial culture by puncture or lavage.1,73,74
CTIt should not be used in the initial diagnosis of ARS, although it is indicated in special situations, such as unilateral signs and symptoms, suspected complications, and treatment failure. It must be considered in severe disease and immunosuppressed patients. Recent studies suggest that routine use of CT in patients with ARS adds little information to their management.1,75,76
Simple X-rayIt has low sensitivity and specificity, being of little use in the diagnosis of ABRS due to the high number of false-positive and false-negative results.1
Ultrasonography (USG)USG of the paranasal sinuses has low sensitivity and very limited usefulness in the diagnosis of ARS, due to the high number of false-positive and false-negative results.1
TreatmentThere is a worldwide concern regarding the indiscriminate use of antibiotics and bacterial resistance. It is estimated that approximately 50 million unnecessary antibiotic prescriptions for RS are given in the US and used to treat viral infections. When a more selective algorithm for antibiotic therapy is followed, the benefit is greater and only three patients need to be treated for one to achieve the expected result.77 Thus, there is a worldwide trend to treat ARS according to disease severity and duration.
Antibiotic therapyMeta-analyses of placebo-controlled, randomized, and double-blinded trials show the efficacy of antibiotics in improving symptoms of patients with ABRS, especially if carefully administered. They are not recommended in cases of viral RS, as they do not alter the course of the disease;78 they are never indicated for symptomatic treatment and their indiscriminate use should be avoided, since that can increase the risk for the development of bacterial resistance.79
Clinical studies have demonstrated that approximately 65% of patients diagnosed with ABRS show spontaneous clinical resolution80 sometimes within the first few days;78 therefore, the initial adjuvant treatment without antibiotics is a viable option in cases of mild and/or post-viral sinusitis. The introduction of antibiotics should be considered when there is no improvement after adjuvant therapy or if symptoms exacerbate. Antibiotics are indicated in cases of moderate to severe ABRS; in patients with severe symptoms (fever > 37.8°C and in the presence of severe facial pain); in immunocompromised patients, regardless of disease duration; and in cases of mild or uncomplicated ABRS that do not improve with initial treatment with topical nasal corticosteroids.81,82
There are no studies that define the optimal duration of antibiotic treatment. In general, treatment duration varies from seven to ten days for most antimicrobial agents and 14 days for clarithromycin. Amoxicillin is considered the antibiotic agent of firs choice in primary health centers, due to its effectiveness and low cost. Macrolides have comparable efficacy to amoxicillin and are indicated for patients allergic to β-lactams.79,82,83 In cases of suspected penicillin-resistant S. pneumoniae, severe cases, and/or associated comorbidities, broader-spectrum antimicrobials are indicated.
Intranasal topical corticosteroidsPatients older than 12 years with post-viral RS, or with uncomplicated ABRS with mild or moderate symptoms81 without fever or intense facial pain,82 benefit from topical nasal corticosteroids as monotherapy. In addition to relieving the symptoms of rhinorrhea, nasal congestion, sinus pain, and facial pain/pressure,81 topical corticosteroids minimize the indiscriminate use of antibiotics, thus reducing the risk of bacterial resistance.82
Studies suggest that topical nasal corticosteroids in combination with appropriate antibiotic therapy results in faster relief of general and specific symptoms of RS, especially congestion and facial pain,84–89 and accelerates patient recovery, even when there is no significant improvement in the radiological image.87,88,90 However, the optimal dose and treatment duration still need to be established.85–88 Although there are no studies comparing the effectiveness of several types of nasal corticosteroids in ARS, many of them (such as budesonide, mometasone furoate, and fluticasone propionate) have shown benefits90 Their use is recommended for at least 14 days to effect improvement in symptoms.
Oral corticosteroidsThe use of oral corticosteroids for adults with ABRS and intense facial pain is recommended, as long as there are no contraindications to their use.91,92 Oral corticosteroids should be used for three to five days, in the first few days of the acute event only, and always associated with antibiotic therapy, in order to shorten the duration of facial pain91 and decrease the need for analgesics.92 Evaluation after ten to 14 days of treatment shows no significant differences in symptom resolution or treatment failure when comparing antibiotic therapy alone and antibiotics with oral corticosteroids.92 The few studies in the literature using oral corticosteroids in the treatment of ABRS showed favorable results with methylprednisolone and prednisone.
Nasal lavageDespite the frequent use of isotonic or hypertonic saline solution in nasal lavage of patients with rhinitis and RS, little is known about their real benefits in ARS.
Randomized trials93 comparing nasal saline and hypertonic solutions showed greater intolerance to hypertonic solution. A meta-analysis of placebo-controlled, randomized, double-blinded trials showed evidence of limited benefit of nasal saline irrigation in adults, with no difference observed between case and control groups. A single study showed a mean difference of improved time to symptom resolution of 0.3 days, without statistical significance.94
In another meta-analysis of patients younger than 18 years with ARS, there was no clear evidence that antihistamines, decongestants, and nasal lavage were effective in children with ARS.95
Despite little evidence of clinical benefit, the use of nasal saline lavage is generally recommended in patients with ARS. It promotes improvement of ciliary function, reduces mucosal edema and inflammatory mediators, and helps to cleanse the nasal cavity, by removing the infectious secretions, and saline lavage has no reported side effects.96
Oral and topical decongestantsThe use of oral decongestants alone or associated with antihistamines in patients with ABRS does not significantly change the clinical or radiological evolution, either in children97 or in adults.98
Topical nasal decongestants (topical vasoconstrictors), such as 0.1% xylometazoline, are not indicated alone for the treatment of ABRS,99 but they do provide subjective and objective improvement of nasal obstruction in patients with viral ARS. In cases of patients with ABRS as a complication of persistent rhinitis, the use of topical nasal vasoconstrictors may relieve nasal obstruction100 and increase inspiratory nasal flow.101 Even in this restricted population, it is important to consider the complications caused by interactions with other drugs, as well as the possibility of adverse effects on hypertension, glaucoma, diabetes mellitus, thyroid disease, urinary retention, and benign prostatic hyperplasia (BPH).99
Due to the rebound effect, the use of topical nasal vasoconstrictors should be restricted to a maximum of five days.
They should not be used by children younger than 2 years.
Nonsteroidal anti-inflammatory drugs (NSAIDs)A systematic review with Cochrane collaboration demonstrated that NSAIDs do not significantly reduce the overall symptom score of patients with common cold, or the duration of colds. Nonetheless, their analgesic effect is beneficial with improvement of headache, ear pain, and muscle and joint pain, and without evidence of increased adverse effects in this population. Therefore, they can be used for the symptomatic improvement in patients with common cold.102
In spite of their analgesic effect in acute inflammatory processes of the ear, oropharynx, and paranasal sinuses,103 NSAIDs are not recommended as the only treatment of ABRS, and should be used with caution even when associated with antibiotics, due to the increase in possible side effects.104,105
MucolyticsThe association of mucolytics in the treatment of ARS is still controversial. It is believed that they reduce nasal secretion viscosity due to their mucoregulatory activity, resulting in fragmentation of acid mucopolysaccharide (AMPS) fibers and, therefore, facilitating mucociliary transport and their elimination through the nose and paranasal sinuses.106 When combined with antibiotics, they may facilitate penetration into the paranasal sinus mucosa and improvement of the inflammatory process.107 There have been some studies using oral bromhexine combined with oral antibiotics and acetylcysteine combined with topical nasal antibiotics.106–108 However, those studies did not clearly state the time and severity of RS; therefore, their results should be analyzed with caution. Studies with oral erdosteine showed no significant benefit in children.109
PhytotherapicsThere are few placebo-controlled, randomized, and double-blinded studies of herbal medicines in the treatment of ARS. In spite of the benefits demonstrated by some of them, their use in clinical practice should be approached cautiously because of the scarcity of published evidence regarding the pharmacokinetics and pharmacodynamics of these components and their mechanisms.
- •
Pelargonium sidoides:110 A study with Cochrane collaboration for the treatment of acute respiratory infections concluded that it can be effective in alleviating the symptoms of the common cold and post-viral ARS in adults.
- •
Myrtle Essential Oil: which is extracted from Pinus spp. (pine), Citrus aurantifolia (lime) and Eucalyptus globulus. A controlled, randomized, multicenter trial reported a statistical difference in symptom improvement score of post-viral ARS (from 10.5 to 9.2) when compared to placebo, reducing the need for antibiotics (20% in patients who used the medication vs. 40% in those who used a placebo). In Germany, it is recommended for the treatment of ARS.111
A Cochrane review112 with ten studies demonstrated that probiotics are superior to placebo in reducing the number of patients with upper respiratory tract infection episodes, number of episodes per participant, and antibiotic use. Therefore, they may be indicated for the prevention of the common cold.
ImmunomodulatorsA systematic review113 of eight randomized controlled trials (RCTs) in children with more than three episodes of upper airway infections per fall/winter (six months) who used OM85 BV extract demonstrated that these children had fewer episodes of upper airway viral infections when compared to the placebo group (38% vs. 52%; p < 0.001), and that the benefits are greater for patients with risk factors for recurrent infections.
Acute rhinosinusitis complicationsRS complications are caused by acute or chronic infections; although they are more common in children, they may also occur in adults and can be orbital-palpebral, bone, and intracranial.
EpidemiologyMost RS complications originate from ethmoid sinus infections. It is estimated that prior to the advent of antibiotics, the rate of blindness arising from complications was up to 20%, and is currently around 11% of cases. Mortality from meningitis of sinus origin in the past was approximately 17%; it currently ranges from 1% to 2.5%.1,114–116 The mortality rate from intracranial complications is around 20% to 40%,114,117 and from neurological deficits, 25%.117,118 The incidence varies by geographic region. In the Netherlands, for instance, the complication rate is estimated at 1:12,000 ARS in children and 1:36,000 ARS in adults,119 whereas in the United States it ranges from 2.7 to 4.3:1,000.000;120 and in France, 2.5:1,000,000/year, excluding pediatric patients.121 It is more frequent in males. In children, complications usually occur from the acute processes, whereas in adults, they are more often seen with CRS with or without polyposis.119,120,122 There are no exact prevalence data for the several types of complications. Orbital complications comprise from 60% to 75%; intracranial, from 15% to 20%; and osseus, 5% to 10%.123 Childhood sinusal disease is the presumed cause of 10% of intracranial suppuration, 10% of preseptal cellulitis, and 90% of orbital cellulitis, subperiosteal and intraorbital abscesses.124 Antibiotic prescription does not appear to reduce the incidence of complications.5,119
PhysiopathogenesisDissemination occurs by direct extension, bone erosion, through diploic veins and hematogenously through venous involvement.125 Certain anatomical characteristics are important in the genesis of these complications:1,114
- •
the thin boney lamina papyracea that separates the orbital contents from ethmoid cells;
- •
in children, a number of larger neurovascular foramina and several boney sutures that remain open in the medial orbital wall and facilitate the dissemination of infection; and
- •
the valveless venous system that allows blood to flow unimpeded into the interior of the skull. The principal pathway is through the superior and inferior ophthalmic veins, which communicate with intraorbital vessels and directly with the cavernous sinus.
Existing classifications are based on anatomical-clinical criteria, but none is universally accepted. It is important to remember that the orbital septum consists of a deflection or extension with change in direction, laterally forming the lateral palpebral ligament, and medially, the medial palpebral ligament, behind the lacrimal sac. It functions as a protective barrier against infections for the internal orbital area.116,118,123 The earliest classification was that of Hubert, which dates from 1937.118 In 1970, Chandler et al.123 proposed a classification that is still the most cited in the world literature, which takes into account the orbital septum:
- •
Group 1 – periorbital cellulitis: eyelid inflammation with edema, without dissemination into the orbit;
- •
Group 2 – Orbital cellulitis: the infection crosses the orbital septum and penetrates the orbital cavity;
- •
Group 3 – subperiosteal abscess: post-septal abscess between the lamina papyracea and the periosteum, contained by the latter;
- •
Group 4 – orbital abscess: true orbital abscess, purulent secretion inside the orbit, within the extrinsic eye musculature, near the optic nerve;
- •
Group 5 – thrombosis of the cavernous sinus.
Due to failures observed in this classification revealed by imaging studies (CT and magnetic resonance imaging [MRI]), Mortimore and Wormald126 suggested removing the cavernous sinus thrombosis group from orbital complications and placing it into the cranial complications group.
- •
Group 1 – preseptal infection;
- •
Group 2 – subperiosteal post-septal infection;
- •
Group 3 – intraconal post-septal infection.
In Brazil, Velasco et al.127 proposed a simpler classification, with only three groups, considering preseptal cellulitis as a palpebral rather than orbital infection:
- •
Orbital cellulitis;
- •
Subperiosteal abscess;
- •
Orbital abscess.
Among all classifications, most authors still use that proposed by Chandler.116,128–131
BacteriologyRegarding the bacteriology in orbital complications, the most common microorganisms are the same that are identified in RS.128 The widespread use of the heptavalent pneumococcal conjugate vaccine (PCV7) has reduced the frequency of S. pneumoniae in RS complications, with a subsequent increase in infections by S. aureus, as well as in the prevalence of methicillin-resistant S. aureus (MRSA) associated with orbital infections.132
Orbital-palpebral cellulitisThe presence of palpebral edema, erythema, localized pain, nasal obstruction, rhinorrhea, difficulty opening the eyes, and possibly fever, can be observed in cases of orbital-palpebral cellulitis. It is caused by venous obstruction created by the pressure on the ethmoid vessels,116,118 and can progress into palpebral abscess and rarely, to cutaneous fistula. Visual acuity and ocular motility are preserved and this assessment is difficult in some children.133 Inflammation of the eyelid and conjunctiva is observed on CT as edematous tissue.134 It occurs as a complication of viral upper respiratory tract infection, acute dacryocystitis, skin infection and, less commonly, RS.135–138 It has a favorable prognosis with antibiotics and often requires no imaging tests, being treated as simple acute ethmoiditis.121
Orbital cellulitisIt is characterized by edema extending into the post-septal region. It appears most often as a complication of acute RS.137,138 It presents exophthalmia, chemosis, and conjunctival hyperemia,130 and affects the orbital adipose tissue without forming an abscess. Visual acuity and ocular motility are usually preserved, but a slight decrease of the latter may occur, and some children initially may lose the ability to distinguish green and/or red colors.126,139,140 Ophthalmologic evaluation and emergency CT are necessary, and treatment should be aggressive and immediate.
Subperiosteal abscessThe clinical picture presents with high fever (39.5°C or higher), chills, changes in general status, exophthalmos with exophoria, decreased ocular motility, severe pain, preserved visual acuity (although decreased in some cases),141 and leukocytosis with a shift to the left.141 The CT discloses the presence of purulent collection in the medial orbital wall, between the periorbital and the orbital bone, with an extraconal location and, thus, outside the ocular muscles.116 The most common microorganisms are Streptococci in children and anaerobic bacteria in adults. Total vision loss can occur, especially in diabetic adults. Abscesses located more superiorly can result in intracranial complications by extending into the frontal lobe.
Orbital abscessIt is an intraconal lesion, commonly the consequence of late diagnosis or immunosuppression.142 The clinical picture is more severe with irreducible, painful exophthalmos with severe chemosis, complete ophthalmoplegia, and marked decrease in visual acuity.130 The CT image shows purulent collection in the soft tissues around the eyeball. It may remain localized or extend through the orbital septum, emerging as a floating mass in the eyelid. It is a severe condition that can lead to amaurosis. The visual impairment depends on the orbital pressure and optic neuritis. Thromboembolism may occur in the vascular supply of the nerve, choroid, and retina. With increasing pressure, there is retinal artery occlusion, which, if lasting over 90minutes, leads to irreversible degeneration of the optic nerve and retina.116,118
The orbital apex syndrome is a localized form of orbital cellulitis, wherein vascular-nervous lesions occur in cranial nerves III, IV, and VI, and in the ophthalmic branch of the V nerve, which pass through the superior orbital fissure and optic foramen.116,118 Clinically, the eyeball is fixed and pupils are dilated and nonreactive to light; ptosis, and palpebral, corneal, and conjunctival hypoesthesia are also observed. When there is a concomitant lesion in the optic foramen, ophthalmoplegia, amaurosis, severe ocular pain, and sensory deficits from anesthesia to neuralgia are seen in the distribution of the ophthalmic nerve. Since the posterior orbital bone is thicker than the anterior bone, these findings are rare and, when present, are more common in sphenoethmoiditis.
Cavernous sinus thrombosisIt consists in the dissemination of an infection along the optic canal or intravenously to the cavernous sinus. It causes blindness, abolition of the pupillary reflex to light, corneal anesthesia, and paralysis of nerves III and VI. The following are also observed: high fever, altered sensorium, prostration, severe deep retro-orbital pain, bilateral involvement, and central nervous system signs. The accompanying photophobia and neck stiffness may be mistaken for meningitis. The mortality rate is approximately 30%.114
DiagnosisThe diagnosis of complications should involve otorhinolaryngologic, ophthalmic, and neurological evaluations, as well as neurosurgical assessment, when necessary. Imaging studies, particularly CT with contrast and MRI, play an important role. High-resolution CT is the technique of choice when orbital complications are suspected. MRI better characterizes the local extent of disease or its dissemination beyond the nasal and paranasal cavities. A combination of CT and MRI is useful in cases of difficult diagnosis.143 It usually discloses swelling of the medial rectus muscle, periorbital lateralization, and downward and lateral displacement of the eyeball. When obliteration of the extraocular muscle detail is evident and the optic nerve appears as confluent mass, an orbital abscess is present. Imaging studies may also detect air bubbles produced by anaerobic bacteria. The predictive accuracy of the clinical diagnosis is 82% and of the CT is 91%.144–146
Laboratory analysis usually shows leukocytosis with a left shift; an elevated CRP level is associated with more severe outcomes and may suggest or indicate the need for more aggressive treatment in the early phase.71
Differential diagnosisPatients with RS and proptosis may have a subperiosteal orbital hematoma; 13 cases have been reported in the literature.147 Orbital lymphatic malformations can lead to proptosis, compressive optic neuropathy, vision loss, and cellulitis. The MRI shows a well-outlined intraorbital mass with a heterogeneous signal.148
General treatment standardsTreatment is medical for orbital-palpebral or periorbital cellulitis. It requires hospitalization, careful observation, and intravenous antibiotic therapy. Clindamycin or amoxicillin + clavulanate potassium with metronidazole and/or, particularly in children, oxacillin + ceftriaxone can be used in the treatment. Most patients respond well to the conservative treatment, and surgical intervention is not necessary.115,116,118 It is always recommended to discuss with the local Hospital Infection Committee which antibiotic is the most appropriate.
The identification of abscesses on the CT, orbital or progressive visual findings, or lack of response to intravenous antibiotics, are all indications for surgical exploration. Intensive ophthalmological control is crucial.149
Children with small and medium-sized subperiosteal abscesses, without significant ocular signs, may be successfully treated medically. Surgical drainage is indicated for medium-sized to large abscesses with severe visual loss, and in cases with inadequate response to medical treatment.150 Usually, a medium-sized subperiosteal abscess that does not improve with medical therapy can be drained endoscopically, while a lateral or intraconal abscess may require an open procedure.151
There are controversies regarding the surgical indication in subperiosteal abscesses. For the initial treatment,141 many studies have documented an improvement in young children with medical therapy alone.133,142,152 If medical treatment is chosen, it is essential that clinical improvement occurs within 24 to 48hours; that there is no visual impairment; that the abscess volume is less than 0.5 to 1.0mL; the abscess is located medially; that there are no systemic symptoms and that the child is less than four years of age.153 Surgical drainage should be strongly considered when an older child has a subperiosteal abscesses with significant ocular findings, when improvement is not observed after 48hours of medical treatment, when the abscess volume > 0.5mL, the length > 17mm, and the width > 4.5mm.154 In general, immediate surgical drainage is indicated in the following situations: the abscess is not in a medial location, or there is visual loss, clinical deterioration or an absence of clinical improvement in 24 to 48hours.114,116,141
Based on the diagnosis of a subperiosteal abscess, in which there is no purulent secretion after opening the lamina papyracea, an orbital abscess should be suspected, and incisions should be performed along the periosteum to release the purulent material from the orbit.155 Some authors always recommend surgical treatment for subperiosteal abscess, with drainage of the abscess and sinuses involved.141 The endoscopic approach is always safer and more effective, but associated external approaches may be necessary.
Acute sphenoid RS may cause thrombosis of the ipsilateral or contralateral cavernous sinus. Early surgical sphenoidotomy and aggressive medical treatment are the bases of the successful management of this life-threatening complication.156
Intracranial complicationsThese include extradural and subdural abscesses, brain abscesses, meningitis, cerebritis, and thrombosis of the cavernous and superior sagittal sinus.120,122,123,131,153,156 The most common are: subdural abscess (56%), epidural abscess (44%), and brain abscess (19%). Multiple intracranial complications were observed in 31% of cases.117,122,157 All clinical forms begin as encephalitis, but as necrosis and liquefaction occur, a capsule develops, forming the abscess. There is a high incidence of anaerobic bacteria and mixed flora. Microorganisms most frequently mentioned in the literature include Streptococcus millieri and S. anginosus, Fusobacterium sp. and S. aureus.114,128,158,159S. anginosus causes more severe infections, higher rates of neurological complications, more neurosurgical interventions, and more central nervous system sequelae.160 Polymicrobial cultures are obtained in 50% of patients.161
MeningitisIn decreasing order of frequency, the paranasal sinuses related to the origin of meningitis, are the sphenoid, followed by the ethmoid, frontal, and maxillary sinus. Clinical manifestations include fever, severe headache, neck stiffness, irritability, and behavioral disorders. CT defines and delimits the disease and can identify the presence of additional complications. Lumbar puncture125 reveals increased proteins and cells, and a culture and sensitivity test should be performed. Lumbar puncture is contraindicated in the presence of intracranial hypertension (ICH) or abscess.125 The treatment is medical, and sinus intervention is reserved for refractory cases. The mortality rate is around 5%.116,118
Extradural abscessThis consists of a purulent collection between the dura mater and the cranium. Occasionally, it is associated with frontal osteomyelitis. The clinical manifestations are vague, with few or no neurological signs, which, when present, include persistent headache, fever, and rarely, behavioral changes. The diagnosis is usually delayed because of a failure to recognize the significance of the clinical findings. By the time of diagnosis there is usually ICH with worsening headache, vomiting, and behavioral changes.116,118
Subdural abscessSubdural abscess is characterized by the presence of purulent collection between the dura mater and the pia-arachnoid. Patients present severe headache, fever, and decreased level of consciousness. CT shows a decreasing image, not extending beyond the midline, thus differentiating from the extradural abscess. Surgery is performed at the neurosurgeon's discretion.116,118
Brain abscessThe incidence of cases of sinus origin varies greatly, ranging from 3% to 11% up to 66%. The most common location is in the frontal lobe. Focal symptoms and increased intracranial pressure appear late with poor general condition, coma, and cranial nerve palsy. The frontal lobe is an area of clinical silence that yields inconstant symptoms. Fever, ICH, seizures, waking period disorders, coma, motor deficit, sensory disturbances, and altered vision may occur.
Imaging studies show a rounded lesion with a hypodense center and peripheral enhancement that initially is irregular but becomes more well defined as the necrotic portion progresses. It can be multilocular. Lumbar puncture is contraindicated due to the risk of brain stem decompression and herniation. The initial treatment during the phase of cerebritis is based on antibiotic therapy, although empirical. Once an abscess is formed, surgical drainage is indicated by puncture or craniotomy159,162,163 combioned with concomitant paranasal sinus drainage.162 The latter, alone, does not substitute for intracranial drainage.157 Antibiotics should be maintained for four to eight weeks.128 Third-generation cephalosporins can be used in combination with with metronidazole, mannitol, hyperventilation, and dexamethasone, with or without anticonvulsants.128
Bone complicationsThey occur with the extension of the infectious process to the bone, possibly involving the brain and nervous system. The most common associated sinus infections are in the frontal and maxillary sinuses.1 In the frontal region, there is a diploic spongy boney layer, with a rich vascular network including diploic veins that course between the external and internal bone laminae. These veins do not have valves and allow unimpeded passage of blood between the spaces of the sinus mucosa and the skull.1 Series of patients with complications of sinusitis demonstrated that osteomyelitis occurs in 9%146 to 32% of cases.164 A peculiar clinical form of localized frontal osteomyelitis may be focal or circumscribed, often with progression to a cutaneous fistula The diffuse or disseminated frontal form is characterized by thrombophlebitis of the diploic veins, which progresses to the frontal bone and the cranial cavity, leading to avascular necrosis, bone sequestration, and expansion to subperiosteal infection. It is more common in young individuals with extensive, pneumatized, and vascular diploe, increasing the risk of infection.165 A softened, floating tumor may be observed, without signs of inflammation called Pott's tumor.118 It corresponds to a subperiosteal abscess of the frontal bone associated with underlying osteomyelitis.117,166 Radiographically, it has three phases: 1) condensation with bone sequestration; 2) rarefaction, when there is bone necrosis; and 3) decalcification or absence of bone tissue in irregular areas, interspersed with islands of calcification and areas of bone sequestration. A CT scan confirms the diagnosis. Scintigraphy with technetium 99 for diagnosis and with gallium 67 for the follow-up is useful, but not essential.165 Treatment consists in the administration of clindamycin and abscess drainage through coronal access, with reconstruction.
Osteomyelitis of the maxillary sinus is often a complication of odontogenic infection, more common in infants.
Atypical rhinosinusitis complicationsThe literature contains case reports with unusual complications, such as lacrimal gland abscess,167–169 orbital hematoma,147 nasal septal abscess,170 nasal septal perforation,171 frontocutaneous fistula,172 clival osteomyelitis with paralysis of the VI nerve,173 acute ischemic stroke,174 and septicemia.175 Cases of children with orbital sequelae after cochlear implant surgery176,177 have also been observed. In one study, 14% of patients showed evidence of RS. The most likely hypotheses were: the patient's position during surgery, duration of surgery, or minor trauma to the lamina papyracea during perforation of the mastoid.177
Chronic rhinosinusitisDefinition and epidemiologyCRS is an inflammatory disease of the sinonasal mucosa that persists for at least 12 weeks. In specific cases, exclusive sinus involvement can be observed, as in odontogenic sinusitis or fungal ball.
CRS can phenotypically be divided further into two main entities: CRS without nasal polyps (CRSsNP) and CRS with nasal polyposis (CRSwNP). Currently, there is evidence to suggest that these two entities have distinct physiopathogenic mechanisms.
CRS is a common disease in the population, and studies about its epidemiological data are important to evaluate its distribution, analyze its risk factors, and promote public health policies. However, such data are scarce in the literature. Additionally, data comparison is hindered by the different definitions and methodologies used in the studies.
This disease has a high direct cost for public health, which includes medical visits, supplementary and radiological exams, hospitalization, surgery, and drug treatment, as well as indirect costs, such as decreased work productivity and absenteeism.178–181 In the United States, the estimated expenditure on these patients is US$ 8.6 billion per year,182 of which US$ 150 million are related to antibiotic use.183 Additionally, overall quality of life and disease-specific questionnaires show great impact of CRS on patients’ quality of life.184–187
In 2007, the European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS)188 was published, and a CRS definition was introduced for epidemiological purposes, characterized by the presence of two or more of these symptoms for more than 12 weeks, a) nasal obstruction/congestion; b) rhinorrhea (anterior or posterior); c) facial pain/pressure; d) reduction or loss of olfaction. One of the two symptoms had to be either a) or b) above. Supplementary examinations, such as nasal endoscopy or imaging studies were not required for diagnosis.
The annual study by the National Center for Health Statistics (NCHS) of the United States population by means of household surveys observed a prevalence of self-reported medical diagnosis of sinusitis of 13% of the adult population in 2010 and a response rate of 60.8%. However, there was no distinction between ARS and CRS in this study, as the criteria that defined CRS in this questionnaire was an affirmative answer to the question: “In the last 12 months, have you had sinusitis diagnosed by a physician or healthcare professional?”189 However, this prevalence is used in most published studies that refer to CRS.
In Canada, an epidemiological study of complex sampling design, with a national response rate of 82%, was performed through telephone interviews with individuals aged 12 years and older, with symptoms of chronic diseases for more than six months.190 Individuals were considered as having CRS when the following question was answered affirmatively: “Do you have sinusitis diagnosed by a healthcare professional?” In that study, the prevalence of self-reported RS was 5%.190
In South Korea, a nationwide study was performed through complex cluster and multistage sampling. A medical team that included an otorhinolaryngologist visited households and performed interviews with participants aged 12 years or older. The diagnosis of CRS was defined by a positive response to symptoms of nasal obstruction and rhinorrhea for more than three months and an endoscopic examination with findings of polyps or secretion in the middle meatus. The estimated CRS prevalence in South Korea was 6.95%.191
Hastan et al.192 published part of the results of the Global Allergy and Asthma Network of Excellence (GA2LEN) European multicenter study related to the investigation of CRS epidemiology. A questionnaire was mailed to a randomized sample of adults between 15 and 75 years in 19 centers in Europe, covering 12 countries, using as a diagnostic criterion the epidemiological definition published in EP3OS 2007 (The European Position Paper on Rhinosinusitis and Nasal Polyps)188 The estimated prevalence of CRS in Europe was 10.9% (6.9% to 27.1%), but the overall response rate was 48%, with wide variation between centers (23.2% to 80.3%).213 Tomassen et al.193 reported the consistency and validity of the epidemiological criterion of CRS defined by EP3OS 2007m188 using data from the GA2LEN study.
Pilan et al.,194 in a recent study in the city of São Paulo, Brazil, with complex sampling design incorporating stratification and multiple selection stages to obtain a representative sample of the population, used the epidemiological definition of CRS recommended by EP3OS The questionnaire involving this definition was applied in 2006, through household interviews, to individuals aged 12 years or older, and a prevalence of CRS of 5.51% and high response rate of 87.8% was observed.194 No statistically significant difference was found in prevalence by gender. In that study, there was a higher prevalence of CRS in patients who had asthma and rhinitis. However, there was no significant association with smoking. For the purpose of comparison with the methodology used in the study by Pleis et al.,189 the same question was inserted: “In the last 12 months, have you had sinusitis diagnosed by a physician?” The self-reported prevalence of RS diagnosed by a physician (with no distinction between acute and chronic) was 16.55%.
PhysiopathogenesisMicrobiologyIn contrast to the etiopathogenesis of ABRS, which involves a continuum of changes promoted by a viral infection followed by bacterial superinfection, the role of microbial agents in the pathogenesis of CRS is not yet fully elucidated.
No microbial agent alone is capable of creating the diversity and heterogeneity of the physiopathogenic processes involved in CRS; therefore, a microbial theory is not always applicable to all patients. Great advances have been made in the last decade, with studies that have explored new interactions between the host and the environment in the genesis of chronic inflammation, opening perspectivesfor new therapies.
Viral participationCurrently, there is little research regarding viral involvement in the physiopathogenesis of CRS. Despite the high frequency of acute infections of the upper airways, it is not very clear yet whether viruses act as a source of chronic stimulation or trigger the initial inflammatory process. As they have the capacity to incorporate into the host DNA as the episomal form, the virus may persist chronically in the respiratory mucosa. Recent studies of viral genomic detection have demonstrated from none195 to significant rates of the main respiratory viruses, especially rhinovirus196 and metapneumovirus.197 However, there is still no evidence whether these viruses are involved in a latent infection without cytopathic effects for the host, or whether they are active, producing antigens and replicating.
Fungal participationAmong the different classifications of sinonasal chronic inflammatory processes involving a fungal etiology, it is indisputable that, in some conditions, such as fungal ball and the invasive chronic forms, the role of fungi is essential.198,199 However, the participation of fungi in the forms of idiopathic CRS, those without apparent cause, is still a subject of much controversy.
The theory of a fungal etiology for CRS200 was heightened by the correlation of the high incidence of the detection of fungi in CRS patients, associated with a high number of eosinophils in tissue and secretions. Several in vitro studies have demonstrated that stimulation of lymphocytes by fungal antigens could produce increased amounts of IL-5, IL13, and IFN-gamma201 and stimulate eosinophil degranulation.202,203 However, other investigators failed to reproduce such findings or even found divergent results.204–206
The attempt to prove the fungal theory through clinical trials with topical and systemic antifungals did not produce encouraging results. Controlled studies have failed to demonstrate the efficacy of oral207 and topical antifungals for the treatment of CRS.208–213
The fact that the ubiquity of fungal elements could act as a constant stimulator of innate immunity receptors and, in turn, could lead to stimulation of specific inflammatory responses cannot be ignored.214,215 In light of the present evidence, fungi appear to have universal participation in CRS, and play a modulatory role in some patients.216
Bacterial participationStudies involving conventional bacterial growth and identification techniques have been widely performed in patients with CRS. Most Brazilian217,218 and international studies219–222 observed a higher prevalence of S. aureus, Gram-negative, and anaerobic bacteria in patients compared to controls, or even those with ARS. However, the identification of bacteria by the traditional method, through in vitro culture, has some sensitivity and specificity limitations. In general, the conventional method only shows positivity for dominant microorganisms or those with favorable growth on that medium, representing only the collection site (middle meatus, nasal cavity, and paranasal sinus) with a risk of contamination from other regions (such as the nasopharynx and the nasal vestibule), or does not allow the differentiation of colonizing microbes from pathogenic microorganisms (for instance, S. epidermidis).
In order to overcome such limitations of flora interpretation in individuals with CRS, more sensitive and specific techniques have been used for the characterization of nasal flora in healthy individuals and those with CRS. Recent studies using molecular techniques have shown high prevalence of bacteria, with a predominance of S. aureus, P. aeruginosa, and anaerobic bacteria, characteristically polymicrobial.223–225 These studies have demonstrated that individuals with CRS have the same bacterial load as their healthy peers, but with lower flora diversity, indicating a possible microbiota disorder.226 Broader studies including the analysis of the human microbiome are still necessary to assess the importance of the quantity and biodiversity of these bacteria in patients and healthy individuals, considering that the genetic, geographic, and environmental characteristics may influence the microbiota in different health scenarios.
Based on conventional microbiology and some molecular studies, it was observed that S. aureus is the main bacterial agent found in Western patients with CRS, both in preand postoperative conditions,227 with a lower prevalence in the Chinese population;228 it is more frequently identified in patients with extensive sinonasal polyps than in controls or even individuals with CRSsNP.229
A peculiar characteristic of S. aureus is its capacity to produce exotoxins with superantigen properties. There is evidence that staphylococcal superantigens may play a role in the physiopathogenesis of CRS, especially in CRSwNP, with induction of specific polyclonal IgE and mast cell stimulation;230 increases in IL-4, IL-5, eosinophils, and eosinophil cationic protein;231–234 and association with severe asthma.230,235–238 However, the mere presence of enterotoxin-producing S. aureus in the nasal cavity is not sufficient to produce a chronic inflammatory reaction and polyp formation.239 It is believed that the primary action of superantigens is to modulate inflammation mation in the upper airways, depending on the distinct reactions of each individual.240
Another bacterial form, which has been demonstrated in CRS, is bacterial biofilms. Despite the great variability in the prevalence of biofilms in different studies, probably due to the different techniques used, it is estimated that at least 25% of cases are associated with their presence.241,242
In general, patients with CRS have significantly higher rates of biofilm when compared to healthy individuals. However, similar to planktonic bacteria, it is unclear what the real role of biofilms is in the physiopathogenesis of CRS and it is not possible to determine whether the colonization of biofilms would be the cause or the consequence of chronic inflammation.243
In addition to the possible involvement of multiple species of bacteria in biofilms, simultaneous fungal and bacterial colonization has also been observed.244
The presence of certain bacterial species in biofilms can diversely influence the outcome of patients undergoing surgical treatment. S. aureus and P. aeruginosa are associated with worse postoperative outcome or a greater number of revision surgeries.245–249 Moreover, patients with biofilms that include H. influenzae or S. epidermidis have better postoperative prognoses.247
In terms of pathogenic mechanisms, two independent studies, using different detection methods for different populations, showed opposite results on polarization of the inflammatory response, whether to Th1 (neutrophils, IFN-gamma, interferon-gamma, macrophage inflammatory protein-1 [MIP-1], granulocyte colony stimulator factor [G-CSF])250 or Th2 (IL-4, IL-5, eosinophil cationic protein [ECP])251 in patients with biofilm. Recent studies have shown that the presence of biofilm is associated with increased positivity of tumor necrosis factor (TNF) receptor expression types I and II and increased plasma cells and eosinophilic markers, both in CRSwNP and CRSsNP.252
In CRSwNP, the presence of biofilms modifies the pattern of antigen-presenting cells in the subepithelial layer, with a possible change in the stimulatory mode of adaptive responses and consequent production of specific inflammatory mediators.253 Finally, the presence of bacterial biofilms in CRS is associated with increased expression of tolllike receptor-4 (TLR-2) and nuclear factor kB (NF-kB), but not TLR4, possibly with activation of innate immunity in different ways than in CRS without biofilm.254
In addition to the bacterial forms that colonize the surface of the sinonasal mucosa, viable intracellular bacteria have also been identified in the respiratory mucosa of patients with CRS, especially S. aureus.255,256
The presence of viable intracellular bacteria could justify another form of bacterial persistence in the respiratory mucosa, especially in chronic and recurrent disease. Although the mechanisms that lead to the internalization and intracellular survival of S. aureus are not known, curiously the intracellular niche of microcolonization is associated with lower adjacent inflammatory triggering, with reduced recruitment of surrounding T lymphocytes and eosinophils.257 Also, Tan et al.258 demonstrated a significant correlation between the intracellular presence of S. aureus with bacterial biofilms on the mucosal surface of individuals with CRS, reporting that both the intracellular persistence and the adhesion of bacterial forms on the surface can contribute to the maintenance of the chronic inflammatory process. Another relevant fact is that the type of strain of S. aureus can also determine the impact on the host. Thus, the capacity to form biofilms on the surface, internalization in specific cells, and the production of certain proand anti-inflammatory cytokines also depend on the morphological and functional characteristics of the bacteria.259
The great diversity of sinonasal microbiota, either as planktonic bacteria, biofilm, or intracellular forms, as well as the numerous possibilities of interaction with mechanisms of innate and adaptive immunity of the host, probably acts as an important factor of tissue inflammation in CRS, either as a triggering or modulating factor or even as a factor that maintains chronic sinonasal inflammation.
Inflammatory mechanismsAlthough similar in their symptoms, CRSsNP and CRSwNP are different at molecular and cellular level. There is growing scientific evidence that the phenotypic differentiation of CRS is insufficient, making it necessary to differentiate between the different types of CRS based on the disease endotype, i.e., the cellular and molecular markers.260 That would be useful not only to better predict patient prognosis but also to develop new therapies, prescribed according to the CRS endotype.
Histologically, CRSsNP is characterized by neutrophil infiltration, increased fibrosis, and collagen deposition in the stroma. The basement membrane is slightly thickened and there are no pseudocyst deposits.261 CRSwNP is characterized by extensive leukocyte infiltration (eosinophilic in 80% of cases) with the overt presence of pseudocysts with albumin accumulation and edema, associated with decreased collagen in the stroma; the basement membrane is thickened and there are significant histological alterations in the epithelium.261
The most recent theories suggest that there is a disorder in the interaction between innate and adaptive immunity in both cases. Adaptive immunity is phylogenetically more recent, coordinated mainly by lymphocytes. This system depends on the individual's prior exposure to this antigen.1
Innate immunity is phylogenetically older and immediately recognizes (without prior exposure) that which does not belong to the body. For example, after one exposure to a single-stranded DNA virus that is not characteristically present in human beings, innate immunity is immediately activated. This system was formerly believed to be extremely rudimentary, but it is now known that it is extremely complex and dynamically interacts with adaptive immunity.
Thus, in simplistic terms, it has been suggested that CRS follows irreparable damage to the epithelium and activation of innate immunity. The latter is ultimately responsible for the activation of the individual's adaptive immunity.262 In this sense, the main cell that initiates this process is the epithelial cell.
The nasal epithelium is important, not only as a mechanical barrier against different pathogens and stimuli, but also as an active participant in the innate and adaptive immune processes.263,264 In ideal conditions, the epithelium is able to destroy these particles without activating the adaptive system.265 Therefore, an epithelial lesion is essential for the chronic inflammatory process.
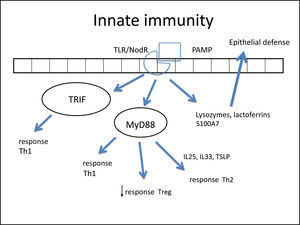
In such an epithelial lesion, the pathogen-associated molecular patterns (PAMPs) bind to pattern recognition receptors (PRRs) present in the cell membrane and cytoplasm of epithelial cells. These PRRs are activated by the presence of pathogens, antigens, and necrotic cells, among other inciters. The best-known PRRs are currently the tolllike receptor (TLR) and NOD-LR-nucleotide-binding and oligomerization domain-like receptors (NLR). TLRs are the most often studied in the nasal epithelium. There are over ten known TLRs, and each is specific for a pathogen. As an example, while TLR-2 binds to Gram-positive bacteria and some fungi, TLR-3 predominantly binds to viruses, and TLR4 to Gram-negative bacteria.
Once bound to PAMPs, TLRs induce the secretion of proteins in nasal mucus (such as lysozymes and lactoferrins),260 cytokines, and chemokines.266 Cytokines are molecules that promote the inflammatory pattern; chemokines are responsible for the recruitment of inflammatory cells toward the injured tissue.
Some studies have reported a decreased secretion of these defense molecules against pathogens (defensin, lysozyme, lactoferrin, S100A7)266–268 in patients with CRS, which impairs the immune function of the epithelial barrier. Additionally, the expression of tight junctions (TJs) is also decreased in nasal polyps.269 The TJs are molecules that bind epithelial cells to each other, control epithelial permeability for the influx of substances or inflammatory cell permittivity, and prevent the entrance of external particles.260 The decrease in expression of these molecules demonstrates epithelial fragility, specifically that of nasal polyps. Both gamma-interferon (IFN-γ, typical Th-1 cytokine) and interleukin-4 (IL-4, typical Th-2 cytokine) can increase epithelial permeability by decreasing TJs.270
There have been reports of changes in the expression of TLRs; while CRSsNP shows increased expression of TLR-2 and TLR-4, nasal polyps have reduced expression of TLR-2 and TLR-9.263,271–273 These changes were especially observed in patients with early recurrence of CRSwNP,272 suggesting the importance of innate immunity in CRS physiopathogenesis.
Once bound to the specific particle, the TLR activates its inflammatory cascade. Essentially, this cascade occurs through its canonical pathway (via myeloid differentiation primary response-88 [MyD88]) or an alternative pathway (via TIR domain containing adapter inducing interferon-β [TRIF]). Both pathways activate transcription factors, molecules that have the capacity to enter the cell nucleus and bind directly to DNA, inducing or repressing gene transcription of some molecules, especially cytokines, chemokines and adhesion molecules. The difference between the two pathways is that the alternative pathway induces the production of IFNs, which triggers the Th-1 adaptive inflammatory response.274–276 The MyD88 pathway triggers the transcription factors nuclear factor κB (NF-kB),274,277 mitogen-activated protein kinase (MAPK), and signal transducer and activator of transcription-3 (STAT-3),260 which amplify the adaptive immune response in some cases with a predominantly Th1 pattern and in other Th2.
In fact, NF-kB is a transcription factor that has an increased expression in patients with CRSwNP.278,279 This factor is especially important, not only for its extensive pro-inflammatory effect, inducing the production of several cytokines such as IL-1β, TNF-α, IFN-γ, eotaxin, (intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion molecule 1 (VCAM-1),278,280 but also because it can directly inhibit the action of corticosteroids in the cell by preventing the binding of its receptor (glucocorticoid receptor [GR]) to the cell's DNA.278,280 A prospective study281 demonstrated that overexpression of NF-kB was related to an earlier relapse CRSwNP. The epithelial cells themselves direct the inflammatory response pattern: examples are IL-33, IL-25, and thymic stromal lymphoprotein (TSLP) cytokines, which induce the polarization of dendritic cells and T-cells to Th2 pattern and, hence, tissue eosinophilia.263,266,282,283 The expression of IL33 that is increased in CRS, has a direct association with the degree of tissue eosinophilia,282 and is present to a greater degree in cases that do not respond to treatment.284 IL-25 and TSLP induce Th2 lymphocyte expansion, regardless of the adaptive response.285,286 TSLP is particularly important for the interface between epithelial and dendritic cells,282 activating them and finally polarizing T cells to Th2 pattern. IL-6 is an essential cytokine for the transition between this phase and the activation of adaptive immunity266 (Fig. 3).
Figure illustrating the participation of innate immunity in the pathogenesis of chronic rhinosinusitis (CRS): once the toll-like (TLR) or nod-like (NLR) receptors bind to pathogen-associated molecular pattern (PAMP), the production of Th1 and Th2 cytokines is stimulated, in addition to the decrease in Treg cytokines through two pathways: myeloid differentiation primary response-88 (MyD88) and TIR domain containing adapter inducing interferon-β TRIF). Furthermore, lactoferrins and lysozymes are produced.
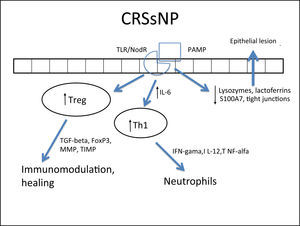
Several cells from the nasal mucosa produce chemokines to attract inflammatory cells and adhesion molecules that facilitate the vascular permeability for the influx of these cells. Together, they increase the influx of inflammatory cells to the site. Examples of chemokines are RANTES (regulated on activation normal T cell expressed and secreted) and eotaxins, which especially recruit eosinophils and are increased in CRSwNP,264,278,287–289 while IL-8 recruits neutrophils and is specifically increased in CRS, with or without NPs.290 With respect to the adhesion molecules ICAM-1 and VCAM-1, results are controversial in the literature, with some studies showing no increase in ICAM-1 expression.288 However, this expression was related to a poorer response to corticosteroids in patients with CRSwNP.291 In cases with CRSsNP, the inflammatory pattern is almost exclusively neutrophilic, mediated by Th1263 with increased IFN-γ, IL-12, and TNF-α283,292 (Fig. 4Fig. 5).
Specific response to chronic rhinosinusitis without nasal polyps (CRSsNP). After stimulation of innate immunity in the presence of high concentrations of IL-6, there is a polarized adaptive response to Th1, with associated increase in Treg. That results in neutrophil response and a modulated inflammatory process.
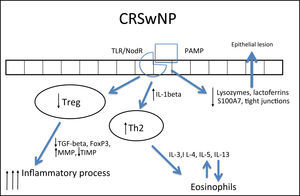
In CRSwNP, there is a predominantly mixed Th1/Th2 inflammatory pattern287,290 in European and American populations, but with a clear Th2 predominance, significant increase in expression of IL-5, in addition to other cytokines, such as IL-4 and IL-13 and GATA-3 transcription factor.1,4,282,283,289,292 IL-5 has particular importance in CRSwNP, as it is primarily produced by eosinophils and its main function is to induce tissue eosinophilia by increasing the influx of these cells and reducing their apoptosis.283,287,290,293 Moreover, IL-5 is associated with increased risk of asthma and other comorbidities,293 as well as a worse postoperative prognosis.293 Eosinophils induce tissue damage, edema, and intense vasodilation by producing proteins such as ECP (eosinophil cationic protein)283,290,292,294,295 and LTs (leukotrienes),296 in addition to producing collagen and thickening the basal membrane in tissue.297 This inflammatory pattern is notably found in patients who have acetylsalicylic acid-exacerbated respiratory disease (AERD, an association of CRSwNP, asthma, and acetylsalicylic acid intolerance).263
In contrast, in patients with cystic fibrosis (CF) and thoseof Chinese origin, nasal polyps are predominantly neutrophilic,263,292 with intense infiltration of IL-8, IFN-γ, myeloperoxidase (MPO), and IL-1β. In the specific case of CRSwNP in Chinese subjects, there is a significant involvement of Th1/Th17 mixed response, with marked increase in the expression of IL-17 by the tissue.260,282,283,292,298
IL-1β expression is increased in polyps, both eosinophilic and neutrophilic. Although it is less significant in the differentiation between Th1 and Th2 patterns, this cytokine is an important pro-inflammatory molecule and its expression is associated with a poorer response to treatment with topical corticosteroids291 and a worse postoperative prognosis.293
In spite of the difference between inflammatory patterns of nasal polyps in Europeans/Americans and Chinese individuals, all share the Treg deficiency.282,298 This is another pattern of T cell response, whose function is to inhibit and contain the inflammatory process. The expression of Fox-P3, a transcription factor that is the main marker of Treg response, is reduced in CRSwNP.282,298,299 Unlike what is observed in CRSsNP, in which the expression of Fox-P3 and TGF-β (transforming growth factor β) was preserved,292 the expression of both of these molecules is reduced in CRSwNP.282,292,298,299 It is currently believed that this is the main difference between the two diseases, as while in CRSsNP the inflammatory pattern is more localized and contained through the maintenance of Treg function, in CRSwNP the inflammatory pattern is diffuse and exacerbated.261,292
In addition to its extremely important role in the containment of the inflammatory process, TGF-β is one of the main inducers of remodeling, a phase during which tissue recently injured by inflammation is regenerated.260,261 While TGF-β is increased in CRSsNP, it is quite decreased in CRSwNP.260,261,283,287,289,290,300,301TGF-βisessentialforthebalance between the expression of matrix metalloproteinases (MMPS),1,282,292 whose essential function is to degrade the extracellular matrix of the polyp stroma (thus contributing to edema) and of its inhibitor (tissue inhibitor of metalloproteinases [TIMP]). MMPs are increased in nasal samples from patients with CRSwNP and CRSsNP,260,261,287,292,297,302,303 facilitating the influx of inflammatory cells. TGF-β, Fox-P3, and TIMPs are increased in CRSsNP but decreased in CRSwNP, which could explain the difference in the extent of inflammatory disease.260,261,292,302,303 The expression of MMP-9 is also related to the recurrence of CRSwNP; therefore, patients with higher expression have a worse prognosis.261,292,303
Despite the advances, there are more endotypes yet to be identified for a full understanding of the pathogenesis of CRS. This knowledge is essential to define the subgroups that are more likely to benefit from one therapy or another. For example, in patients with CRSwNP mepolizumab (anti-IL-5), would be more appropriate for use in patients with increased IL-5 and macrolides for patients without marked eosinophilia.260 The endotyping study of CRS is essential for the development of new, more effective therapies.
Genetics in chronic rhinosinusitisGenomics analyzes the alterations in DNA sequence (genetic polymorphisms) in two ways: studies based on hypotheses and studies free from hypotheses.1,304,305 Most of the published articles addressing CRS are studies based on hypotheses (or candidate gene) that investigate mechanisms or pathways already known to be altered in the disease. A few polymorphisms are analyzed at a time and the cost of research is relatively low304,306 The more well-known studies free from hypotheses are linkage studies and genome-wide association studies (GWAS).304,306–309 GWAS use expensive high-density chips, and can evaluate over one million polymorphisms simultaneously. One way to decrease the costs of that type of evaluation (but not without loss of genetic information) is to perform pools of DNA from cases and controls using just one chip for each group, a strategy known as pooling-based GWAS (pGWAS).304,306–309
Evidence from genetic research in chronic rhinosinusitisGenetic basis for chronic RSThe initial idea of a genetic basis for the existence of CRS came from the existence of familial aggregation. A study of the descendants of two brothers, one with and one without CRS, showed a higher prevalence of CRS in the first group.310 Other studies have also shown familial aggregation,311–314 with concordance between family prevalence and disease severity,311 increased chance of positive CRS family history among patients with CRS,314 and a report of monozygotic twins with CRSwNP, even though they lived in different regions.315
HLA system genesMutations in the human leukocyte antigen (HLA) genes are strongly associated with inflammatory diseases, but the association with CRS is not fully understood.1,304 Alterations were identified in the frequencies of several HLA alleles.316–320 A study of Mexican mixed-race individuals with CRSwNP associated the HLA-DRB1*04 allele to the disease,319 but this was not observed in a Turkish population with CRSwNP, except for CRSwNP with asthma or AERD.318 These findings show that this allele may be associated with different CRS phenotypes in different populations.
CFTR geneIt is located on chromosome 7q and its mutations are the cause of CF. It is the gene most often related to CRS. There is a correlation between homozygosity of the ΔF508 mutation and the presence of polyps in CF in the Brazilian population.321 The presence of CF mutations (even without CF) is a risk factor for CRS,322–324 and patients with CRS without CF are more likely to carry mutations in the CFTR gene than controls; the ΔF508 are those most frequently identified followed by M470V mutations.325
Innate immunity genesCandidate gene studies with TLR-2 polymorphisms did not find any association between CRSwNP and TLR2 R753Q rs5743708 polymorphism;326,327 however, there is an association with TLR2 polymorphisms rs3804099 and rs380410048. The polymorphism of the gene for the bitter taste receptor TAS2R38 appears to influence the ability of cells to fight respiratory infections and may be an unknown component of the innate immune response, acting as a sentinel for infections by Pseudomonas aeruginosa.328 CD14 is a component of innate immunity, whose C-159T polymorphism in its gene was associated with CRSwNP.329 Nitric oxide (NO) is a molecule whose function is the defense against bacterial biofilms produced by NO synthase (NOS). Sixteen polymorphisms in the NOS1 gene and its ligand were assessed, and the polymorphisms rs1483757 and rs9658281 may exert a protective effect against CRS.330 The microsatellite polymorphism CCTTT in the NOS2A gene was associated with CRSwNP when it presented 15 or more repetitions.331
Other genes involving inflammation remodeling, and metabolismThe T allele of the polymorphism C-590T of IL-4, an important Th2 IL, appears to exert a protective effect against CRSwNP, but the same allele increases the expression of IL-4, instead of decreasing it.332 The G allele of G-174c polymorphism of IL-6 was associated with asthma and CRSwNP.333,334 The AA genotype of polymorphism A-1510C and the CC genotype of the C-1055T were associated with CRS in patients with asthma and acetylsalicylic acid intolerance.335 IL-33, another IL associated with Th2 response, was also associated with CRSwNP, through its A allele of polymorphism rs3939286.336
The C-1562T polymorphism in the MMP-9 gene appears to be associated with CRSwNP,337 although another study only observed an association in CRSwNP with asthma and acetylsalicylic acid intolerance.338 The T allele of the polymorphism of the TGF-β gene C-509T was associated with CRS in acetylsalicylic acid-intolerant asthmatics and was related to lower levels of protein.339 Furthermore, there is a genetic association between polymorphisms TGF-β C-509T and IL-10 A-1082G; mutated alleles are more associated with CRS.340
The leukotriene C4 synthase enzyme is critical for the regulation of cysteinyl-leukotriene synthesis, which is increased in CRSwNP mainly in AERD, and the C allele of rs730012 polymorphism of its gene was more associated with CRSwNP.341,342
Studies free of hypothesesThe binding study on CRS confirms the role of the CFTR gene in CRS. The highest binding peak was found in the connecting region 7q31.1 to 7q32.1, where the CFTR gene is located.307 However, genotyping of 38 mutations of this gene did not reveal which was responsible for this signal.307 Probably, this failure is due to the large number of different mutations in this gene that are not covered by the usual tests.
The first pGWAS study used one chip for over 550,000 genetic polymorphisms, and observed associations with several genes not previously linked with CRS, such as basal membrane and extracellular matrix genes (for instance, laminin-α2 [LAMA2] and laminin-β1 [LAMB-1]) genes of mitochondrial function (for instance, prolyl-tRNA synthetase [PARS2]), and genes for the degradation of lipopolysaccharides (for instance, acyloxyacyl hydrolase [AOAH]).308 The association with AOAH was confirmed in a replication study for CRSsNP, but not for CRSwNP.343
The second pGWAS study was a secondary analysis of the data according to disease severity. In this analysis, there was an association of the G allele of TP73 gene polymorphism (rs3765731) with risk of more severe CRS.309 All these new findings implicated in pGWAS studies require replication and functional validation of polymorphisms.
Final considerationsSome points should be considered for future genetic studies of CRS. Firstly, adequate phenotyping is important in order to prevent mixing different physiopathological mechanisms. Another focus should include replication of results. Moreover, gene-environment interactions should be analyzed; tobacco and pollution are two environmental factors that deserve consideration. GWAS need to be further explored in the research of CRS, as well as other techniques, such as epigenomics and the complete sequencing of genomes and exomes. Finally, it is necessary to develop functional studies. Only then it will be possible to infer that a certain genetic alteration actually interferes with or causes the disease.
Clinical diagnosisSeveral clinical tests have been developed for the clinical diagnosis of CRS, but in most patients diagnosis is based only on the presence of sinonasal signs and symptoms with over 12 weeks of evolution.1,188,344,345 Sinonasal endoscopy and CT are complementary examinations and help in disease classification. In both CRSwNP and CRSsNP, the main symptoms are:
Nasal obstruction344,345An extremely subjective symptom, it is one of the most frequent complaints in clinical practice, affecting approximately 83.7% of patients;192 it is more important in patients with sinonasal polyposis. It is caused by congestion of sinusoidal vessels, resulting in local edema, followed by tissue fibrosis and, ultimately, only improves with the use of vasoconstrictors. Despite being a subjective symptom, several articles in the literature validated nasal obstruction as an important symptom of CRS346 using acoustic rhinometry and peak nasal inspiratory flow.
RhinorrheaIt can be anterior or posterior, secretions can vary from hyaline to mucopurulent, and is present in 63.6% of patients with CRS. It can also be associated with cacosmia, cough, and hoarseness. It is a symptom of difficult validation or quantification192
Olfaction alterationsHyposmia or even anosmia is common, especially in CRSwNP and is present in up to 46% of patients.192,345 It can be caused by an obstructive process (polyps), edema, and/or mucosal degeneration, or be caused by local surgical interventions and can result from a chronic inflammatory process with or without the presence of nasal polyps1,188,347 There are several studies in the literature that clearly and reproducibly demonstrate alterations of olfaction in patients with CRS.1,66
Facial pain or pressureA symptom with variable prevalence (18% to 80%).1 It is more frequently associated with CRSwNP, patients who have difficult t-to-control allergic rhinitis, or during the flare-up processes.1 The rhinogenic headache is a diagnosis of exclusion, according to the International Headache Society (IHS).1
CoughIn childhood, cough is a frequent symptom; it is usually non-productive and may be the only manifestation of CRS. In addition to the usual symptoms, complaints such as hoarseness, pharyngeal-laryngeal irritation, dysphonia, halitosis, ear fullness, adynamia, and sleep disorders should be questioned.1,188,344,345 During anamnesis, it is important, in addition to the abovementioned classic symptoms, to include questions about systemic diseases and predisposing factors that may favor the development of CRS. Personal habits such as smoking, cocaine use, exposure to toxic inhalants, type of climate in the region where the patient resides, and environmental pollution should be investigated.
Physical examinationAnterior rhinoscopy (with and without vasoconstrictor) is of limited value, except in cases of polyposis, when it can be visualized by simple inspection of the nasal vestibule. Nonetheless, it is important to describe signs such as hypertrophic inferior and middle turbinates, septal deviations, or mucosal degeneration. It is noteworthy that there are no pathognomonic signs of CRS.1,344
OropharyngoscopyRegardless of the color, the presence of retropalatal mucopurulent secretion justifies the symptom of postnasal discharge.1,344,345
Complementary examinationsNasal endoscopyNasal endoscopy enables the systematic visualization of the nasal cavity (lower, middle, and upper turbinate), the nasal septum, and the nasopharynx and drainage pathways; it can be performed with or without topical nasal decongestant. The presence of polyps, mucosal degeneration, secretion, crusts, structural alterations, scars and nasal tumors can also be observed. It can be performed at baseline and at regular intervals (e.g., three, six, nine, and 12 months) to aid diagnosis, to supervise disease follow-up and postoperative period, as well as to collect material for supplementary tests.348,349
It is important to perform a systematic assessment of the nasal cavities, such as analysis of the nasal septum, turbinates, and visualization of the middle meatus, of the sphenoethmoidal recesses, and the nasopharynx. It is also necessary to verify the presence of crusts, ulceration, septal perforation, signs of nasal bleeding, and secretions, and to exclude the possibility of associated polyposis and expansive lesions. It is very important to perform the endoscopic assessment in patients undergoing surgery. The evidence of mucosal disease six months after surgery should be considered as CRS. Another factor to be taken into account in patients with previous surgery is the recirculation of mucus by not including the natural ostium of the maxillary sinus in the antrostomy.
Fokkens et al.1 established an endoscopic score for CRS monitoring, as shown in Table 1.
Endoscopic score for CRS follow-up (Source: adapted from Fokkens et al.)1
| Characteristic | Initial assessment | Follow-up 3/6/12/24 months |
|---|---|---|
| Polyp – LNC (0, 1, 2, 3) | ||
| Polyp – RNC (0, 1, 2, 3) | ||
| Edema – LNC (0, 1, 2) | ||
| Edema – RNC (0, 1, 2) | ||
| Nasal discharge – LNC (0, 1, 2) | ||
| Nasal discharge – RNC (0, 1, 2) | ||
| Postoperative | ||
| Synechiae - LNC (0, 1, 2) | ||
| Synechiae - RNC (0, 1, 2) | ||
| Crust - LNC(0, 1, 2) | ||
| Crust - RNC (0, 1, 2) | ||
| Total |
The scoring is performed as follows:
- •
Polyps (0=absent; 1=only in the middle meatus; 2=originate from the middle meatus, but do not completely obstruct the nasal fossa; 3=completely obstruct the nasal fossa);
- •
Edema / synechiae / crusts (0=absent; 1=mild; 2=severe);
- •
Nasal discharge (nasal secretion; 0=absent; 1=clear, fluid; 2=thick, purulent).
Nasal endoscopy is an examination of utmost importance to assist in establishing the diagnosis, supervising disease follow-up in the postoperative period, as well as collecting material for supplementary tests.
Imaging assessmentCT is the imaging method of choice for CRS evaluation; however, it is not the first step in the diagnosis, except in cases with unilateral signs and symptoms and suspected complication. There are several staging systems described in literature, but the most commonly used is the Lund-Mackay system that established the staging based on opacification of paranasal cavities and the ostiomeatal complex350 (Table 2).
Tomographic staging – Lund-Mackay system. (Source: adapted from Fokkens et al.)188
| Paranasal sinuses | Right | Left |
|---|---|---|
| Maxilla (0, 1, or 2) | ||
| Anterior ethmoid (0, 1, or 2) | ||
| Posterior ethmoid (0, 1, or 2) | ||
| Sphenoid (0, 1, or 2) | ||
| Frontal (0, 1, or 2) | ||
| Ostiomeatal complex (0* or 2*) | ||
| Total score for each side |
Score: 0 = no abnormalities; 1 = partial opacification; 2 = total opacification;
0* = not occluded; and 2* = occluded.
It is noteworthy that incidental abnormalities are observed in up to 50% of CTs of “normal” patients.188 Therefore, the diagnosis of CRS based on the CT alone is not appropriate. When compared with CT, MRI is a better test to define soft tissues, and allow for the differentiation of secretions and tumors. Thus, it is a test that complements the CT in patients with suspected neoplasia.
Other examinationsBacterioscopy/sinus secretion cultureIndicated in cases refractory to treatment and when the collected material is not contaminated. It is performed through puncture of the maxillary sinus through the canine fossa and through the endoscope to perform the collection in the middle meatus.351
Nasal cytologyMore often used in the presence of an associated allergic condition. Its use alone does not confirm the diagnosis of CRS.
BiopsyIt is important for the study and classification of the inflammatory state of CRS and sinonasal polyposis. It is indicated for the differential diagnosis of autoimmune and granulomatous diseases, and to rule out neoplasms (especially in unilateral cases).
Mucociliary functionMucociliary function can be evaluated by mucociliary clearance (saccharin test or radioisotope particles), study of ciliary beat frequency, electron microscopy, and nitric oxide measurement.352–354 The saccharin test can give false-positive results. Scanning transmission electron microscopy is important for the diagnosis of primary ciliary dyskinesia.
Nasal patencyNasal patency can be assessed by the peak inspiratory flow, rhinomanometry, acoustic rhinometry, and rhinosterometry. However, it does not define the diagnosis of CRS.355,356
Olfaction assessmentThe evaluation of olfaction can be performed with threshold and quantitative tests. The smell identification test of the University of Pennsylvania was recently culturally adapted to the Brazilian population and validated in Brazil.67
Acetylsalicylic acid sensitivity testWhen AERD is suspected, patients should be advised not to take the drug, as the challenge test that confirms the diagnosis may carry risks for the patient. In the USA, the most common challenge test begins with the ingestion of 30mg of acetylsalicylic acid, increasing the dose until a reaction occurs. Subclinical sensitivity to that drug is probably between 5% and 15%.
Laboratory testsSeveral laboratory tests can be performed in specific cases as part of the differential diagnosis. The clinical history and physical examination determine what tests are requested, that among others include: complete blood count with eosinophils, CRP, ESR, assessment of renal, liver and thyroid function, markers of humoral immunity (immunoglobulins, IgG, IgE subclasses, specific antibodies for tetanus, hemophilus influenzae, pneumococcus, aspergillus), response to immunization, and markers of cellular immunity (T and B lymphocytes), human immunodeficiency virus (HIV), ANCA (antineutrophil cytoplasmic antibodies), and measurement of chloride in sweat.357
Allergological assessmentIt is crucial for patients with a positive history of allergy, AERD, or even with suspected fungal infection.
CommentsThe diagnostic investigation of CSR is based on the patient's natural history, signs and symptoms, endoscopic examination, and CT. The latter is considered as a major factor in the analysis of disease progression and the decision-making of surgical intervention.
More studies are necessary to demonstrate the involvement of predisposing factors in the pathogenesis of CRS, such as: environmental, genetic factors, allergies, LPRD, and immunological and ciliary dysfunctions. The presence of Helicobacter pylori does not preclude screening, by the otorhinolaryngologist, of diseases associated with CRS refractory to treatment.
Social habits are another factor that must be taken into account. Recent studies have demonstrated midline destructive lesions induced by cocaine with ANCA (+) mimicking Wegener's granulomatosis associated with maxillary sinusitis. This finding opens up reflection on the relevance and complexity of the subject, importance of the multidisciplinary study and the social impact in the cause/effect association of CRS.
Associated factors and diseasesCRS has a multifactorial cause that results in persistent inflammation Current knowledge of its pathogenesis does not identify one solitary inflammatory pathway that explains the entire process, from the initial lesion to the structural changes in sinonasal tissue.358 However, there is an emerging consensus that the persistent inflammation that defines CRS results from a dysfunction of the host-environment binomial, which makes apparent the imbalance of external agent interaction, predisposition of the sinonasal mucosa, commensal flora, potential pathogens, and exogenous stress.359
This section will discuss the main diseases and factors associated with CRS, sometimes overlapping, sometimes tangential, as conditions that trigger, exacerbate, or perpetuate persistent inflammation.
Predisposing factors and associated diseasesPredisposing factors and associated diseases to CRS can be grouped into three broad and overlapping categories:
Environmental, local anatomical, and systemic factorsEnvironmental exposureExposure to toxins such as tobacco, ozone, sulfur dioxide, and particulate air pollutants (e.g., smoke from diesel combustion), has the potential to trigger epithelial injury and exacerbate airway inflammation.360 Exposure to air pollution, several chemical irritants, inhalants used in photocopying, and smoke from forest fires1,361 are related to increased prevalence of RS and asthma.362,363 A comparative study among individuals who work in an environment with air conditioning and natural ventilation showed a positive association with increased nasal and nasal-ocular symptoms, persistent cough, and symptoms of RS in those exposed to artificial air conditioning.364
SmokingChildren of parents who smoke are more prone to acute respiratory disease compared with children of nonsmoking parents.46 The adult population also shows a higher prevalence of RS in smokers (53.1%) when compared with nonsmokers (26.4%). Subjects with allergic rhinitis exposed to tobacco have more episodes of respiratory disease when compared with control groups.1
Anatomical factorsAnatomical abnormalities such as septal deviation, concha media bullosa, deviations of the uncinate process, Haller cells, hypertrophic ethmoid bulla, and prominent agger nasi cells are correlated with CRS. These anatomical variations may play a role in the pathogenesis of CRS and increase the risk of sinus mucosa disease.365,366 However, some studies have shown that these anatomical changes are not correlated with CRS.367–369 It is observed that there is no specific study in the literature that correlates anatomical variations with obstruction of ostiomeatal complex drainage and CRS. Although there is no causal evidence that anatomical variations are responsible for CRS, many sinus symptoms improve with surgical correction, which that improves drainage of secretions, and favors sinus ventilation. Therefore, in patients with CRS, it is important to evaluate the anatomy of the nasal cavity.
Odontogenic infectionsOroantral fistula, periodontal disease, periapical abscess, and tooth roots that project into the maxillary sinus are causal factors of acute maxillary sinusitis. In recent years, complications of dental implants were also shown to be the cause of infections. Although the odontogenic causes of sinusitis are common, they are rarely mentioned in recent guidelines and are neglected by many otorhinolaryngologists, dentists, and radiologists.370
The pain is often sinusal and isolated, without nasal involvement. It is most commonly located in the infraorbital region, unilaterally or bilaterally, and may worsen with postural changes of the head. It may also radiate to the forehead, to the maxillary premolar and molar regions. In addition, patients complain of fever and thick retronasal secretion. In cases of purulent nasal discharge despite the use of antibiotics and persistence of infraorbital pain, odontogenic sinusitis should be suspected. On physical examination, there is pain on palpation of the anterior wall of the affected maxillary sinus or the bony prominences adjacent to the first molars.
In some cases, no alterations were observed in the external dental structure and there were no signs of tooth decay.371
PCDPCD is a rare autosomal recessive disorder, in which the cilia are immotile or have an altered pattern of movement, causing failure of mucus transport in the airways. The incidence of immotile cilia syndrome ranges from 1 in 15,000 to 1 in 30,000.
PCD is associated with bronchiectasis and chronic upper airway symptoms such as nasal secretion, episodes of facial pain and anosmia. In neonates, there is continuous rhinorrhea since the first day of life.1 The diagnosis should be suspected in children with atypical asthma, bronchiectasis, chronic productive cough, thick continuous nasal discharge, and severe chronic otitis media (especially in children with continuous aural drainage despite placement of tympanostomy ventilating tubes). Diagnosis is suggested by below normal nasal nitrous oxide levels, and a saccharin test > 30min.372
More specific tests in specialized centers include examination of cilia by electron microscopy. The most common structural abnormalities are the absence of external dynein arms or the combination of absence of both the internal and external dynein arms.41
Kartagener's syndrome is a subgroup of PCD inherited as an autosomal recessive disorder. The structural abnormality is the absence of dynein arms. Situs inversus of organs is found in approximately 50% of cases of dyskinesias.
Young's syndrome is unusual, being a combination of obstructive azoospermia of the epididymis, which is associated with infertility.
Laryngopharyngeal reflux (LPR)The association of LPR with RS is controversial, requiring further studies for confirmation. In children, gastroesophageal reflux disease (GERD) has been associated with RS in many studies. Phipps et al.373 performed a prospective study of 30 pediatric patients with chronic RS who underwent 24-hour pH monitoring and observed that 63% of children with CRS had GERD. Among children diagnosed and treated for LPR, 79% showed improvement in signs and symptoms of RS.373 Although other studies also describe similar results between LPR and CRS, routine antireflux treatment is not recommended for CRS patients.
AllergyReview articles suggest that atopy predisposes to CRS.374,375 Both conditions are frequently associated and share an increasing prevalence.376,377 A number of studies report that atopy markers are more prevalent in populations with CRS.35,378–380 However, the role of allergy in CRS is questioned by other epidemiological studies, which showed no increase in the incidence of infectious diseases.381
Between 0.5% and 4.5% of subjects with allergic rhinitis have NPs.382–384 Conversely, the prevalence of allergy in patients with NPs ranges from 10% to 64%.385–387 Contrary to studies that reported atopy as more prevalent in patients with NPs, other authors found no such association.384,387–389 Allergy does not appear to have a direct association with NPs, but can be an aggravating factor.
Recently, Bachert et al.390 found an association between levels of both total and specific IgE and eosinophilic infiltration in NPs.
A recent study391 that compared the bacterial flora of the nasal mucosa between allergic and nonallergic individuals, did not demonstrate greater bacterial growth in those with allergies. Nonetheless, in allergic rhinitis, there is no doubt that the mucosal edema in the region of the sinus ostia may compromise ventilation and sinus drainage, causing mucus retention and increasing the risk for infection.
Non-allergic eosinophilic rhinitisNonallergic eosinophilic rhinitis has similar clinical characteristics to those of allergic rhinitis. Its onset occurs in adulthood, around the age of 20-30 years, and it is often associated with RS with polyps and 30% of patients also have asthma. It shows normal skin test and IgE levels. The use of acetylsalicylic acid and other NSAIDs is not recommended.
AsthmaCRSwNP and asthma are frequently and closely related, but their interaction is still not well understood.392,393 Asthma occurs in 26% of patients with CRSwNP compared with 6% of controls.394,395 Alternatively, 7% of asthmatic patients have NPs,382 with a prevalence of 13% in non-atopic asthma and 5% in atopic asthma.388 There is a high prevalence of radiological abnormalities of the paranasal sinuses in asthmatics.396–398 Asthmatic individuals with CRSwNP have more severe nasal symptoms. This combination should be a clinical clue to suggest severity in both diseases.399
Acetylsalicylic acid intolerance or acetylsalicylic ac-id-exacerbated respiratory disease (AERD)Acetylsalicylic acid intolerance, asthma, and NPs are frequent associated, and then are known as Samter's or Widal's Syndrome. It commonly initially presents with only NP and asthma with acetylsalicylic acid intolerance appearing later. The etiology is unknown, although its evolution is well known there is a very high rate of recurrence and frustration after surgical treatment.
Thirty six to ninety six percent of patients intolerant to acetylsalicylic acid have CRSwNP.35,383,400–404 Radiological evaluation of these patients reveals that up to 96% have alterations in the paranasal sinuses.405 From an epidemiological viewpoint, patients intolerant to acetylsalicylic acid are usually non-atopic, with an increased prevalence after forty years of age. Children of patients with asthma, NPs and acetylsalicylic acid intolerance had NPs and CRS more often than children of the controls,406 suggesting a hereditary factor. Zhang et al.407 found that the enterotoxin-specific IgE can be found in most NPs of patients with acetylsalicylic acid intolerance.
Cystic fibrosisThe finding of NPs in a child is rare (less than 0.1% of children) and should elicit an investigation for CF, which is present in up to 60% of children with NPs. In contrast, NPs do not affect all patients with CF, and the presence of NPs varies with the particular mutation of the CF gene responsible for the disease, but, in general, approximately 20% of CF patients have NPs. CRS is present in 70% to 100% of the patients. The clinical manifestations of CF are variable, and adults in their fifth and sixth decades of life can, on rare occasions, be newly diagnosed with CF.408,409
Adults with NPs dating from childhood should get tested for sodium/chloride in sweat or genetic testing or both to evaluate the presence of CF. Up to 7% of patients with CRS are heterozygous for a CF gene, when compared with less than 1% of normal controls. The physiopathological implications of this finding remain unknown. CRSwNP in CF is usually characterized as non-eosinophilic and more neutrophilic CRS.410
ImmunodeficienciesPrimary or congenital immunodeficiencies manifest with symptoms of RS since childhood, usually associated with infections in other organs. In acquired immunodeficiency, especially in HIV-positive patients, RS is quite common, being present in over 50% of patients. Immunosuppressed patients after immunotherapy treatment or patients with diabetes mellitus may have CRS, and can have either indolent or fulminant invasive fungal RS, which are usually severe and should be promptly treated after they are diagnosed.
Concomitant chronic diseasesA number of diseases can be associated with CRS; due to their broad involvement with general practice, it is impossible to exhaust the subject. Sometimes the sinonasal complaints can offer the first suggestion to attain an important clinical diagnosis, positively contributing to patient evolution. They are:
- •
Wegener's granulomatosis: associated with vasculitis, it often starts in the nose and paranasal sinuses with CRS, and at advanced stages, disseminates to other organs, especially the kidneys and lungs. The diagnosis is difficult and requires biopsy confirmation of vasculitis; the possibility of a positive biopsy is increased when performed in an affected maxillary sinus region.
- •
Sjogren's syndrome: mainly characterized by dry eyes and mouth; it is associated with CRS and rheumatologic symptoms.
- •
Churg-Strauss syndrome: CRSwNP, severe asthma, and vasculitis are observed. Widespread eosinophilia has also been reported; a complete blood count showing an increase of more than 20% in eosinophils is a very suggestive finding.
- •
Systemic lupus erythematosus: it is sometimes difficult to diagnose, depending on a number of criteria, but it is often associated with CRS.
- •
Sarcoidosis: often associated with CRS, it is also difficult to diagnose, showing non-caseous granuloma at biopsy. At anterior rhinoscopy, fine slightly yellowish granules are a suspicious finding that suggests that other tests necessary to establish the diagnosis be performed.
Other systemic diseases are associated with CRS, and patient management is easy when a diagnosis has already been established, but the challenge of finding a possible systemic cause of CRSor a factor for its recalcitrance is an obligation of the otorhinolaryngologist.
CommentsThe multiple causes of CRS can result in isolated sinonasal manifestations, but it is important to note that the nose and paranasal sinuses may reflect the first symptoms of systemic diseases. The identification of predisposing factors and diseases associated with rhinosinusites are of utmost importance for adequate patient management.
Clinical treatmentTreatment with systemic and topical antibioticsThe growing perception of CRS as a multifactorial inflammatory process has been clearly expressed in the latest consensus, i.e., it is not a persistent bacterial infection.411 This fact has led to an obligatory theoretical reassessment of the use of antimicrobials for treatment of this entity. However, and unfortunately, it is not surprising that, in practice, antibiotics remain a constant part of the drug arsenal used in these patients’ everyday life, and is persistently present in different proposals for disease management.412 This is possibly due to the lack of awareness of the absence of bacteria both in free form and/or in biofilms in the paranasal sinuses of these patients. This main theoretical basis for the choice of antibiotics also suffers from the inability to differentiate the true role of bacteria found in the paranasal sinuses, because their identification alone does not mean the presence of an infectious condition or inflammatory reaction to their presence.413 However, the identification of bacteria, such as Staphylococcus and Pseudomonas, in higher percentages among patients with recurrent conditions (postoperative) perpetuates the belief about the need to consider them as part of the physiopathogenesis of CRS. In spite of those statistically significant results, it must be highlighted that, in terms of percentage, the number of positive cultures in that study was higher both in the group with a poor outcome and in those with a favorable outcome (87% vs. 73%); for the bacteria in question, the absolute difference was 14% (39% vs. 25%).414
Recent studies have investigated bacteria as a necessary element responsible for maintaining the balance of inflammatory response, depending on their interaction with the host. The topical use of probiotics and bacteria in an attempt to create flora and biofilms that can induce nasal homeostasis is an example.415 In the past five years, there has been no truly remarkable evidence for the use of antimicrobials in CRS. Despite this lack of evidence, there are still recommendations for the use of macrolides for long periods, for instance, in the absence of elevated serum IgE.1,416–420 Meltzer et al.,421 in a review article concluded that there is a lack of publications that establish a proven effective proposal for the treatment of CRS. The authors point out that, as long as the different presentations of the disease are not well defined, several different treatments will follow, with limitations regarding interpretation and extrapolation of results.
They also stated there are signs of increased interest in developing research protocols, but the identification of ongoing studies of RCTs versus placebo (i.e., adequate designs to search for such responses) at the United States National Institute of Health (NIH-ClinicalTrial.gov) does not support that claim (http://clinicaltrials.gov/ct2/ results). Thus, more specific inclusion and exclusion criteria, randomization, prospective design, and control groups are required for the study of antibiotic treatment in CRS.
Systemic antibioticsFew studies have evaluated the use of systemic antibiotics in patients with CRS with and without NPs. They have been studied mainly in relation to their effects during flare-ups of chronic conditions. Undoubtedly, the most common longterm use (over four weeks) is due to the anti-inflammatory effect exhibited by some drugs in this class, such as macrolides.
Van Zeele et al.422 studied doxycycline in the treatment of patients with CRSwNP compared to methylprednisolone (20 days) in an RCT versus placebo design. During the 12-week follow-up period, the antimicrobial showed less dramatic results than oral corticosteroids, but the effects persisted longer, both with respect to endoscopic characteristics (size of polyps) and the measurement of inflammatory markers that were different than those of the corticoid. These findings lead to the hypothesis that the drugs might have a synergistic effect when used concomitantly. In another study, albeit observational with 125 patients, the authors found no evidence of the effectiveness of antibiotics, as they also deduced that typical findings, on endoscopy and CT, are nonspecific and are not compelling as an indication for these drugs.423 Still following the line of treatments with antimicrobials for a short period of time (21-30 days), Shlalek et al.424 studied different antimicrobials (ciprofloxacin, amoxicillin/clavulanic acid, and co-trimoxazole) in patients with polyposis, and observed no statistically different results. If there is consensus that the bacteria may be part of the etiology, but certainly are not the main factor in CRS, studies such as that by Liu et al.,425 become significant. Unfortunately, with a sample of only six patients, the authors studied the effects of treatment on the microbiota of the maxillary sinus of patients with clinical picture and findings consistent with persistent disease in this sinus, even after surgical treatment. In addition to describing individual variations in the flora, they observed significant changes, such as the emergence of less susceptible bacteria.
In the 2008 guidelines,411 the possibility of using of some antibiotics, identified as having anti-inflammatory effects, appeared to be the start of a new era for some patients with CRS. However, the lack of definitive studies and the increased risk of inducing bacterial resistance brought on new questions. Videler et al.426 conducted an RCT versus placebo study to evaluate azithromycin for 12 weeks in 60 patients, with and without polyposis, with and without asthma, as well as a percentage who had already been submitted to surgery, assessing several objective and subjective outcomes, none of which showed statistical differences compared to the placebo group. The comparison of the characteristics of this sample in relation to the sample from a previous study, also RCT versus placebo, which showed significance in favor of the treated group, indicated the possibility that this type of treatment benefits a specific population of patients with CRS (without polyps, with normal IgE and, possibly, with less mucosal disease).427 In another retrospective study, Videler et al.428 observed improvement in some outcomes among Dutch patients with CRS with both azithromycin and trimethoprim-sulfamethoxazole, when compared to those who did not receive any antimicrobial drug. The authors noted that, considering the type of design, it would not be appropriate to define the true impact of these drugs.
In the same year, Majima et al.429 compared the efficacy of clarithromycin for 12 weeks or associated with clarithromycin plus carbocysteine in 425 patients, and concluded that the combination of the two drugs yields significantly better results compared to the use of antimicrobials alone. While they had a large sample and significant results, the lack of a placebo group significantly hindered the extrapolation of their results.
CommentsThe authors warn about the frequent use of antibiotics and the importance of knowing how to differentiate them among the therapeutic options for CRS. Nonetheless, there is not enough information to completely eliminate their use. It is necessary to find ways to identify exactly the patients who could benefit from antimicrobial use in cases of unequivocal clinical flare-ups and to identify the specific bacterial agent through culture and sensitivity test. Regarding the extended use in CRSwNP cases in which there is persistence of severe symptoms without improvement and without serum IgE elevation after multiple treatments (including surgery), there is still not enough evidence; their possible biological effects must be significantly considered when restricting their use.
Topical antibioticsIn light of the difficulties in CRS management, treatment with topical solutions has drawn the attention of the scientific community in the last decade, hoping for a new improvement in therapeutic results. With respect to the assessment of systemic antibiotics, the problems regarding the quality of the available literature are similar. There are problems not only with the appropriate study design (RCTs versus placebo), but also with the choices of the populations studied. Among the topical options are antibiotics, which are part of the available treatments for patients with chronic lower airway diseases.
In addition to the effectiveness of these drugs in their topical form, other issues must be addressed. One concern is the possible adverse effects from an unknown degree of systemic absorption. For instance, gentamicin, although given in low concentrations, was detected in serum after lavage during a sinonasal surgical procedure.430 Another concern, rarely addressed but nonetheless important, is the possible impact on the microbiota in terms of the induction of resistance. Finally, it is necessary to know whether the drugs used topically actually reach the paranasal sinuses. In a cadaver study only small particles (0.67 to 0.99 microns) reached the maxillary sinuses with large antrostomies. Larger particles were deposited on the nasal valve.431
Few studies have addressed quality of life improvement in patients with CRS treated with topical antibiotics delivered by a small-particle nebulizer, and some did not find different results from those obtained with saline solution lavage, and cautioned against the possible adverse effects related to the absorption of these drugs.432 More recently, a clinical trial by Videler et al.433 did not find any statistical difference between nebulized colimycin and bacitracin in patients previously submitted to a surgical procedure who were resistant to other treatments.
In conclusion, there is no evidence for recommending the use of topical antibiotics for CRS with and without NPs.
Corticosteroids in chronic rhinosinusitisIn CRS, whether with or without nasal polyps, there is only one consensus at the moment: it is an inflammatory disease with different triggers. Therefore, no choice is more rational than the use of drugs with anti-inflammatory effects, whose main representatives are corticosteroids. The potential of these drugs as modulators of bacterial presence has also been investigated. This group of drugs includes options for topical intranasal, oral systemic, and injectable use. The indications include continuous symptomatic control, surgical preparation, and postoperative maintenance. The surgical procedure-related use will be discussed in another section of this document. The use of these medications will be divided between patients with and without NPs. This division is justified because they are two distinct groups in terms of physiopathogenesis, symptoms and therapeutic results.1
Topical corticosteroids (CRSwNP)These medications favorably alter the cytokine profile in the subepithelial layer, but do not effect any change in the characteristics of the biofilm.434
Goggin et al.435 demonstrated the in vitro inhibitor potential of bacterial growth in the form of biofilm when exposed to topical corticosteroids. In a systematic review of 48 studies, it was observed that early surgical treatment is a beneficial factor for topical corticosteroid effectiveness, especially by helping with drug distribution in the sinonasal mucosa.436
In another systematic review, 25 studies on the use of these drugs in patients with CRS and polyposis showed that these drugs were effective, but the same effectiveness was not seen in patients without polyposis.437 Similar results were reported in another meta-analysis.438 In a recent Cochrane review, topical corticosteroids were effective in improving symptoms and reducing polyp size and the rate of post-surgical recurrence. The authors also observed that patients submitted to previous sinonasal surgery responded better to this therapy. They also reported there was no difference regarding adverse effects compared to the placebo group.439
The major adverse effects of topical corticosteroids, in addition to varying degrees of local irritation, are epistaxis and rarely septal perforation.1 It is also worth mentioning that in the international literature there are discussions about the benefits of different techniques of distribution and devices used with the drugs.436,437
Topical corticosteroids in CRSsNPUnlike patients with polyposis, the results in the group without polyps are not as homogeneous; there are reports of positive effects on symptoms, but not on other outcomes, such as endoscopic scores and radiological aspect.440
Systemic corticosteroids in CRSwNPA recent systematic review recommended that these drugs be used only for a short period and for preoperative preparation.441 EPOS 2012 also discussed the beneficial effects in relation to symptoms and polyp reduction, but since this is a chronic disease, it is worth mentioning that the duration of these beneficial effects was brief.1
Systemic corticosteroids in CRSsNPThe same recent systematic review suggested that there is no evidence that either indicates or contraindicates the use of systemic corticosteroids in this subgroup.441 EPOS 2012 corroborates this opinion, reflection the available literature.1
CommentsTherapy with topical and/or systemic corticosteroids is an important part of CRS treatment. This effect is demonstrated most convincingly in patients with polyposis. Although more studies are required to support this claim, they are considered as allies in the fight against CRS in general, especially when used topically. Systemic administration is suggested for cases of CRS with uncontrolled symptoms, in which the goal is to decrease, even temporarily, the impact of the disease on the patient's life. In these situations, it is recommended to use the lowest effective dose for the shortest possible time, to minimize the potentially more severe side effects.
Antileukotrienes and chronic rhinosinusitisNasal polyposis (NP) is a chronic inflammatory disease of the upper respiratory tract that affects 2% to 4% of the population and two thirds of patients with acetylsalicylic acid-sensitive asthma. The histology of polyps is similar to that seen in polyps of patients with asthma, and is characterized by abundant eosinophils, mast cells and high levels of pro-inflammatory cysteinyl leukotrienes.1
It has been suggested that one potential cause of CRSwNP is any potential defect in the eicosanoid pathway that is strongly associated with acetylsalicylic acid intolerance.442 Specifically, the increased synthesis of pro-inflammatory leukotrienes and decreased synthesis of anti-inflammatory prostaglandins have been the accepted mechanism, not only for CRSwNP in acetylsalicylic-acid-sensitive polyposis, but for acetylsalicylic-acid-tolerant individuals as well.443
Regarding leukotrienes and CRS, there is abundant data demonstrating their role in reducing inflammation, especially with respect to eosinophils and the eicosanoid pathway.444 The use of montelukast showed a reduction in eosinophilic inflammation, viability, and cytokine production in nasal polyps after treatment with montelukast.445
Di Capite et al.446 used immunohistochemistry, immunoassays, and cytoplasmic calcium ion imaging in human mast cells from cultures acutely isolated from patients with polyposis. They demonstrated that calcium influx into the mast cell through the activation pathway of calcium channel release stimulates the production of leukotrienes C4, which, in turn, activates greater calcium influx. The combination of low concentrations of calcium release activated channel blockers and leukotriene receptor antagonists was as effective as the concentration of the two antagonists alone to inhibit mast cell activation. This fact should be further studied in order to assess whether there is any clinical significance in this mast cell inhibition of antileukotrienes.446
The data regarding the pathophysiology of CRS very clearly support the use of antileukotrienes, but when the data from randomized, double-blinded clinical trials on the effectiveness of leukotriene inhibitors are analyzed they do not support theoretical studies as clearly.447
After more than 15 years of their use, the effectiveness of antileukotrienes in allergic rhinitis and asthma has been clearly demonstrated. Montelukast showed good effectiveness for the treatment of seasonal and perennial allergic rhinitis in large, double-blinded RCTs, indicating significant improvement in nasal and ocular symptoms between one and three days, as well as in nocturnal symptoms, quality of life, and sleep.448
The allergic rhinitis and its impact on asthma (ARIA) initiative suggests that leukotriene receptor antagonists are effective, well tolerated and very safe; they are recommended for use in adults and children with intermittent allergic rhinitis and in preschool children with persistent allergic rhinitis.449 Leukotriene antagonists such as montelukast, zafirlukast, and zileuton were evaluated in studies involving several patients with CRSwNP and AERD,450,451 but the results were not definitive. Many were open uncontrolled studies that suggested a benefit of leukotrienes regarding symptomatology, the size of the NPs, and the tomographic scores.452 Other results include significant score improvement in symptoms of headache, facial pain and pressure, ear discomfort, dental pain, purulent nasal discharge, postnasal drip, nasal congestion with obstruction, as well as olfaction.453 These authors also concluded that leukotriene-modifier drugs, when added to standard medications, including corticosteroids, result in nasal symptom improvement in patients with CRS with and without polyposis.454–456 However, data from a double-blinded RCT did not consistently support the benefit of antileukotriene therapy in patients with CRS.450,457 Although they are effective in patients with AERD, that effect is no greater than is seen in acetylsalicylic-acid-tolerant individuals.456,458
The combination of montelukast with intranasal corticosteroids has been shown to be effective in CRS. According to Fergusson et al.,459 montelukast added to intranasal corticosteroids improves symptoms in patients with CRS, with excellent safety profile A recent study evaluating the postoperative effects of montelukast and intranasal mometasone in patients with CRSwNP showed complementary results between the drugs. Both treatments led to a significant reduction in SNOT-22 scores and NPs, with a marginal benefit of montelukast alone.460 For these reasons, leukotriene action, when analyzed from the perspective of evidence-based medicine, reveals limited level of efficacy (III) and low grade of recommendation (C) for patients with CRSwNP.461
CommentsInitially used in the treatment of asthma and then allergic rhinitis, antileukotrienes have been used for more than 15 years, and have proven efficacy, level of evidence A and recommendation for these diseases.449 Later, they were used in other chronic nasal diseases, mainly CRS with or without nasal polyps, because of the high morbidity and the low quality of life of affected patients.
Montelukast has been the most often used antileukotriene to date, and there are data demonstrating its action as a leukotriene receptor antagonist. Its anti-inflammatory actions, mainly those related to eosinophils, and its cytokines have been demonstrated by several studies. Another important factor related to montelukast is its high safety and tolerability, being free of adverse effects, even in children.444 What is clearly concluded is its usefulness in allergic patients with asthma and in those with acetylsalicylic acid intolerance. These are the patients with CRS that should use antileukotrienes as treatment, whether as an adjunct therapy or not, in the postoperative period, and as maintenance therapy.
According to Scadding et al.,462 over time, it becomes apparent that certain patients respond better to antileukotrienes than others. The reasons for this fact are becoming gradually clearer,462 and genetic characteristics are being associated with these responses. The pharmacogenetic tests required to identify patients who might benefit more are not yet available. Therefore, a simple therapeutic test for approximately one month, with monitoring through objective and subjective measures is suggested, especially in patients whose treatments with other medications have shown limited response. Patients who may present with Churg Strauss syndrome are the exception.
Acetylsalicylic acid desensitizationAcetylsalicylic acid desensitization in cases of CRS has been a clinical treatment option in patients with AERD. This disease is characterized by CRS with polypoid degeneration, asthma, and acetylsalicylic acid intolerance. Patients have a high rate of disease recurrence, depend on corticosteroids, and have a high rate of recurrent nasal surgery, or require NSAID therapy for other medical reasons, such as chronic arthritis or coronary disease.463 Desensitization can be performed through the nasal, inhalation, and injectable routes. The proposed desensitization therapy was first described in 1976 and was supported by the observation of improvement of sinonasal symptoms with the use of acetylsalicylic acid for the treatment of other systemic diseases.464,465
Desensitization methodologyThe drugs used for desensitization are acetylsalicylic acid and lysine acetylsalicylate. The improvement obtained with this process is dose dependent and after maintenance dose discontinuation, nasal symptoms recur within a few days. Regardless of the route, there is no pre-defined dose for the start of desensitization or for its daily maintenance. The protocols are based on controlled and progressive administration of small and increasing doses of acetylsalicylic acid ingested within two to three days; when the patient can ingest 650mg and exhibit no adverse reactions three hours later, they are said to be tolerant. The procedure should always be performed in a hospital setting, due to potential occurrence of severe adverse reactions, which can occur in 12.5% to 23% of cases.464,466,467 Maintenance is performed by daily administration of doses of 100 to 300mg.
Standard desensitization is performed with the use of acetylsalicylic acid administered orally for three to five days, and thereafter, the dose should be maintained daily.468 Due to gastrointestinal effects, approximately 30% of patients discontinue treatment, but treatment withdrawal decrease to 9% when proton pump inhibitors are used concomitantly.464,468 Lysine acetylsalicylate administered through nasal route has been employed in more recent studies and carries a lower risk of side effects, as shown in the study by Miller et al.,469 that evaluated 150 patients; only three had an adverse reaction to a dose higher than 375mg of acetylsalicylic acid. When using lysine acetylsalicylate through the inhalation route, the adverse effect is usually moderate bronchospasms, reversible with beta-agonists; when the intravenous route is used, there is no higher incidence of adverse effects than with the other routes, with the advantage of discontinuing the infusion if severe adverse effects occur.470 Any of the routes used require monitoring due to the risk of these severe adverse effects, which may occur after ingestion of from 30 to 150mg of acetylsalicylic acid.464 The addition of intranasal ketorolac in the desensitization test was effective, safe, and achieved the desensitization dose of acetylsalicylic acid more quickly. In the assessed protocols, ketorolac is used in four increasing doses at 30-minute intervals and then oral acetylsalicylic acid is introduced.471,472
Mechanism of desensitizationThe desensitization mechanism is based on the blockade of the lipoxygenase pathway. A decrease in leukotriene B4 and thromboxane B4 has been demonstrated in a cultured cell model after desensitization, as has a reduction in bronchial responsiveness to leukotriene B4 after desensitization has started.473 Eosinophils activation has also been described following intranasal desensitization with lysine acetylsalicylic acid, which is maintained both in the initial and late phases. Cysteinyl leukotriene was also increased in the initial phase, but it did not remain so in the late phase.474 Rizk suggests the possibility of the inhibition of an intracellular biochemical pathway involving anti-inflammatory interleukins IL-4 and IL-13.464 In a recent study, it was postulated that acetylsalicylic acid ingestion reduces the activation of tyrosine kinase, which inhibits the phosphorylation of STAT6, reducing the production of IL-4, with consequent decrease in the production of cysteinyl leukotriene (Cys-LT) and the expression of CysLT receptor, resulting in inflammatory tissue reduction.475 The authors have found significant symptom improvement when desensitization was continued for a period of one to six years, with significant decreases in the use of oral corticosteroids for asthma, the dose of nasal corticosteroids, the number of infections, the need to undergo nasal surgery per year, the number of hospitalizations and improvement in olfaction in these patients.463
The effectiveness of desensitization was also demonstrated in the assessment of polyp recurrence rate. In the group of patients undergoing desensitization, the recurrence rates at one and six years were 6.9% and 65%, whereas in the group without desensitization it was 51.3% and 93.5% respectively. There was a dramatic difference in cost due to the low cost of acetylsalicylic acid and impressive reduction of hospitalizations and surgeries.463,476
In conclusion, desensitization is a treatment option for cases with increased morbidity, and can result in significant improvement for most sinonasal symptoms compared to its use for asthma; the use of acetylsalicylic acid lysine and intranasal ketorolac may contribute to the reduction of adverse effects of desensitization. However, further studies should be performed to standardize the methodology and the acetylsalicylic acid maintenance dose to be used.
AntifungalsAlthough several studies have demonstrated the coexistence of fungi in the nose, both in healthy individuals and in those with CRS, there are still some who recommend the use of topical antifungals for the treatment of this disease.477 The use of topical nasal amphotericin B was associated with lower NP recurrence and improvement in symptoms and computed tomography findings, especially when administered together with lysine acetylsalicylate, according to studies without a control group.432,478–481 Nasal irrigation with amphotericin B suspension twice daily for four weeks showed resolution of the NPs in 39% of cases.432,479 However, subsequent controlled-group studies, using topical amphotericin B (irrigation or spray) did not demonstrate significant beneficial effect for patients with CRS with or without polyposis. There was no difference in inflammatory markers in the control group with the use of antifungals, and healing was better in the control group.212,432 A randomized double-blinded study highlighted the higher rate of adverse effects and the lack of benefit with antifungal treatment.482 Another double-blinded, randomized, multicenter study also showed no difference in symptom improvement after treatment with amphotericin B for three months when compared to placebo.211
Recently, three meta-analyses agreed that, although the use of topical antifungal improved the radiological appearance, symptoms did not improve significantly, and a high rate of side effects was observed.213,432,483–486 Some studies, which used increasing frequency and amounts of topical amphotericin B (four times daily for eight weeks) did not achieve additional effects when compared to the exclusive use of nasal saline solution in the treatment of CRS.208,210 The use of a systemic antifungal, such as oral terbinafine, showed no improvement even in cases of CRS with the presence of fungus in the investigation and a positive culture.207
Studies have also shown bias due to a lack of standardization regarding the type of administration, drug dose, and length of treatment, which ranged from four to 80 weeks.432 Moreover, the type of storage used for amphotericin B was questioned, as this drug loses its stability when exposed to light.432 Thus, Shin et al.201 conducted a prospective, double-blinded, placebo-controlled RCT using amphotericin B for 12 months, and found no significant benefits in 33 patients with CRSwNP.
Currently, the use of antifungal therapies in CRS is very limited.207,212,213,432,477,478,483–487 Sacks et al.485 performed a placebo-controlled RCT to evaluate the treatment of CRS with topical and/or systemic antifungal, and showed that the side effects outweigh the benefits of treatment with antifungal agents. However, other recent studies have found, through PCR analysis of sinonasal cavity secretion, a large volume of unusual types of fungi (A. alternata, C. cladosporioidie, C. herbarum, P. brevicompactum, P. crustosum, and P. chrysogenum) only in patients with CRS.483,488 The authors reported that there are subgroups of patients with high concentrations of these fungi and others without fungi.488 Furthermore, they report that in vitro studies did not test for the entire available antifungal spectrum and the tests were not performed for these unusual species. Therefore, more than one antifungal may be needed in order to eradicate the fungi found in the sinonasal mucosa.483,486 The authors also discussed the possibility of antifungal treatment failure in CRS due to incomplete eradication of the fungi in the nasal cavity.483,488
Khalil et al.484 evaluated antifungal treatment in recurrent allergic fungal CRS after endoscopic sinusectomy using an oral antifungal agent (itraconazole for three months) and/or antifungal nasal spray (500mL fluconazole in saline solution) and the same topical antifungal agent through nasal irrigation. Their results showed that the recurrence rate was similar between patients who received placebo and oral itraconazole (67%). The group that had the fewest recurrences was the one that used fluconazole spray (10%), suggesting that when allergic fungal RS (AFRS) is suspected, it would be appropriate to obtain a culture for fungi, even with a negative result for fungi in the anatomopathological assessment. Moreover, they comment that some authors recommend the use of topical antifungals in combination with topical nasal corticosteroids in AFRS.489,490 However, other studies contradict the use of topical antifungals, reporting that their use may even worsen symptoms and cause deleterious side effects, including the nephrotoxicity and hepatotoxicity seen with systemic antifungals. Some authors have observed good results with itraconazole in refractory AFRS.491,492 Rains and Mineck493 defend the use of a combination of itraconazole and oral and topical steroids with endoscopic surgery for the control of AFRS, considering that, in refractory cases, there may be minimal fungal invasion in the sinonasal mucosa. The authors concluded that the topical antifungal fluconazole (spray or irrigation) can significantly reduce CRSwNP recurrence after endoscopic sinusectomy. In their opinion, there is no benefit in adding systemic treatment with itraconazole,493 because the systemic antifungal agent does not reach the target since the fungus lies on the mucosal surface without penetrating it, and therefore is not reached by blood; i.e., the fungus acts by triggering an allergic and noninfectious reaction.493 For some authors, the conventional treatment with topical corticosteroids and antibiotics in patients during the postoperative period of AFRS shows reasonable benefits in preventing polyp recurrence.484,493 The results correlated well with the findings of a study by Jen et al.,494 who observed that 12 of 16 (75%) patients with allergic fungal RS (AFRS) improved after treatment with fluconazole nasal spray. Moreover, Weschta et al.482 performed a randomized, double-blinded, placebo-controlled trial with topical antifungal amphotericin B in 60 patients with CRS, with ineffective results.
Due to the controversial results of these studies, it is believed that there are different genotypes and, according to environmental and behavioral influences different CRS phenotypes are generated. Therefore, fungi ceased to be the main agent in the physiopathogenesis of CRS, and exert limited influence in the AFRS subgroup. Both the treatments with topical and systemic antifungals in CRS have not shown evidence of their effectiveness in the current literature.432,483–494
Preoperative period in patients with surgical indicationsThe procedures used before CRS surgical procedures are not homogenous and there are few evidence-based publications.
Combinations of antibiotics, steroids, nasal lavage, and nasal decongestants are the most commonly used medications/procedures in the preoperative management. The use of antibiotics to prevent postoperative infections has been adopted in endoscopic sinonasal surgery (ESS), in which 77% of the cases have potentially pathogenic species in the nasal cavity, such as S. aureus, Klebsiella spp., and/or E. coli. Moreover, patients with CRS usually receive repeated courses of antibiotics, which favors the development of resistant pathogenic species. Although antibiotics are widely used by surgeons preoperatively, aiming to decrease inflammation by minimizing infection and therefore improving the operative field, there are no clinical trials justifying this practice, and only a few studies have assessed the postoperative management.495
According to Portela et al.,496 27% of the surgeons prescribed antibiotics preoperatively, whereas 35% did so postoperatively. The drugs used were amoxicillin-clavulanate, clarithromycin, erythromycin, and doxycycline, among others, for a period ranging between seven to 40 days. A recent survey among members of the American Rhinologic Society found that approximately 90% of otorhinolaryngologists prescribed antibiotics for three to four weeks in the treatment of CRS.497
In some institutions, such as Stanford University and the Johns Hopkins Hospital, patients are advised about the possibility of using antibiotics preoperatively, in the presence of active infection. In Brazil, the University of São Paulo (USP) protocol proposes amoxicillin-clavulanate, five to seven days before surgery, for patients with severe inflammatory infections.498–500
Maier and Strutz501 designed a study with 106 patients, including some submitted to parotidectomy and neck dissection. Thirty-six patients in the sinonasal endoscopic surgery subgroup were randomly administered a single dose of 1.5g cefuroxime intravenously preoperatively or that drug plus three additional doses every eight hours postoperatively. There were no reports of infection or side effects for any patient in the ESS subgroup from either treatment arm.
Intraoperative prophylactic antibiotic therapy is also controversial. Some guidelines501,502 for antibiotic prophylaxis in surgery, such as that by the Surgical Infection Society-Latin America, do not recommend the use of antibiotics in endoscopic surgeries. In Brazil, however, first-line hospitals recommend the administration of prophylactic antibiotics. Cefazolin is effective against methicillin-susceptible Staphylococcus and Streptococcus (except pneumococcus and some Gram-negative bacilli), and is the first choice for the prophylaxis of potentially contaminated surgeries. Clindamycin can be used in patients allergic to beta-lactam. Amoxicillin-clavulanate is the first choice for surgeries with risk of contamination by anaerobic microorganisms. Antibiotic prophylaxis must be used during anesthesia. The dose should be repeated if the operating time exceeds the halflife of the antibiotic or if major bleeding (10% to 20% of blood volume) occurs, requiring no further doses postoperatively.495,503-506
Regarding preoperative use of corticosteroids, both oral and topical administrations have well-documented effects on CRS, especially in bilateral nasal polyposis. Nonetheless, there is no standardization of dose and/or type of oral corticosteroid to be used. Some studies have shown the effectiveness of preoperative corticosteroids on operative field improvement, reduced bleeding and surgical time.507–509
Wright and Agraval507 performed a double-blinded, placebo-controlled RCT in patients undergoing endoscopic surgery with CRSwNP. Participants were randomized to receive 30mg of prednisone or placebo for five days preoperatively and nine days postoperatively. There was no difference between the groups with respect to postoperative symptoms.
Another randomized clinical trial evaluated the effect of a single dose of prednisolone (1mg/kg/dose 24h prior to surgery) versus five days of prednisolone (1mg/kg/day before surgery), and preoperatively, in 80 patients with bilateral NPs. The patients underwent surgery under general anesthesia, using the same protocol. Mean arterial blood pressure was 70-80mm Hg in both groups. Mean bleeding during the operation was 266.5 ± 96.31mL in group A and 206 ± 52.81mL in group B, with a significant difference between groups. There was no significant difference between groups regarding the surgeons’ opinion on the quality of the operative field. In conclusion, in contrast to the single 1mg/kg/ dose of prednisilone, treatment with that dosage for five days can reduce blood loss during surgery and improve the quality of the operative field.508
A double-blinded, randomized study analyzed 70 patients with CRS with and without NP. Of that group, 35 received mometasone furoate (MF) and the other 35, a placebo for four weeks prior to ESS. Bleeding in the group treated with MF was 142.8mL, less than that in the control group (170.6mL). The difference between groups was 27.7mL, which was statistically significant The time of surgery was 59minutes in the MF group and 70minutes in the control group. The difference was 11.2minutes, which was statistically significant. The quality of the endoscopic surgical field was significantly better in patients treated with MF. The use of the topical corticosteroid MF preoperatively can improve endoscopic vision, reduce bleeding, and decrease time of surgery in patients with CRS with and without polyps undergoing endoscopic surgery of the paranasal sinuses.509
CommentsAlthough there are controversies in patients with CRSsNP with purulent secretion, amoxicillin-clavulanate 875mg, 12/12h, or cefuroxime 500mg, 12/12h, for seven to ten days can be used preoperatively and maintained for seven to 21 days postoperatively. In some cases, fluoroquinolones and macrolides may be prescribed.
In patients with CRSwNP, the use of oral corticosteroids for three to five days (e.g., prednisone 0.50mg/kg/day) is suggested, which is continued postoperatively according to extent of the disease.
Irrigation of the nasal mucosa with isotonic saline and hypertonic saline solutions, with and without preservatives, is a classic and safe measure in the treatment of CRS; it is very useful to mobilize secretions and to promote mucosal hydration preand postoperatively. However, there is no evidence for a beneficial action if it is used alone.411
Preoperative evaluation according to patient age and comorbiditiesBecause they increase coagulation time and thus increase bleeding, patients are advised to stop taking acetylsalicylic acid, ibuprofen, and other NSAIDs, vitamin E, ginko biloba, ginseng, and garlic tablets seven to ten days preoperatively, If the patient is anticoagulated, it should be discontinued seven to ten days prior to endoscopic sinus surgery, if at all possible. The partial thromboplastin and prothrombin time should be normal. Asthmatics patients or those with other comorbidities should be compensated preoperatively. For instance, patients with asthma may maintain bronchodilator sprays until the day of surgery. Those using steroids chronically can receive 100mg of intravenous hydrocortisone at the immediate preoperative period. CT is essential in surgical planning, and must be able to be viewed in the operating room. The patient should be instructed regarding the procedure to be performed, the steps of the surgery, and the inherent risks of the procedure. and this should be documented by a signed informed consent.
Surgical treatment: techniquesSeveral surgical techniques have been described for use in patients with CRS with and without nasal polyposis who are refractory to medical treatment. There is no single gold standard technique that can be applied to all cases. Due to the lack of RCT, several aspects of the surgical management remain controversial. The most important of these is the extent of surgical dissection. As a result, based mainly on case series studies and expert opinions, current guidelines suggest that surgical management should be individualized. The current trend in CRS with and without nasal polyposis is that the surgical dissection proceed as far as the extent of the disease.1
The most commonly used surgical approach is the endonasal access route. However, some cases may require an external or a combined access routes, such as lesions of the lateral maxillary sinus, the frontal sinus, or when there are no reliable anatomical landmarks for an exclusive endonasal approach. Regardless of the technique and the instruments used, there is clearly a learning curve in ESS. The surgeon should have deep knowledge of the surgical anatomy and should have undergone training in specific courses on dissection of the nose and paranasal sinuses.
Surgical treatment of CRS has advanced greatly with the use of sinonasal endoscopy. The image accuracy provided by the endoscopes (0 degree wide angle lens), as well as their angulations (30, 45, and 70 degrees), allows for the visualization of all the details and recesses of the paranasal cavities. Moreover, the development of other specific equipment and tools for intranasal and sinus use (e.g., dilation balloons, neuronavigator, and microdebrider) allow performing surgical procedures ranging from simple dilation of drainage ostia to complete marsupialization of the paranasal sinuses into the nasal cavity.510–512
The following paragraphs will briefly review the main endoscopic techniques described for the surgical treatment of CRS.
The most widely used technique for the surgical treatment of CRS is the functional endoscopic sinus surgery (FESS).513 However, the word “functional” was questioned, and many now prefer the term endoscopic sinus surgery (ESS), which we will use hereafter. The word “functional” was previously used by authors to differentiate the procedure from the traditional techniques, which promoted complete removal of paranasal sinus mucosa for the treatment of CRS. The FESS technique, as originally described by Messerklinger,513,514 aimed to improve ventilation of the paranasal sinuses and, consequently, mucociliary function, while preserving the mucosa as much as possible. Judicious removal of inflamed tissue and bone to clear and, where necessary, extend the natural drainage ostia are the basic tenets of ESS.
The surgical techniques employed by surgeons are quite variable, especially the surgical instruments, the anatomic landmarks, methods for hemostasis, the sequence and especially the extent of dissection. Most surgeons follow the anteroposterior lamellar dissection, as systematized by Stammberger et al514 that begins with the unciform process, then the ethmoid bulla, followed by the basal lamella, and the sphenotomy. Others have as main parameters the outer limits of the dissection, that is, the lamina papyracea laterally, the middle turbinate medially, and the skull base posteriorly. This is the centripetal technique.515
The way surgeons handle the sinus ostia, especially of the maxillary, frontal, and sphenoid, as well as the intrasinus inflammatory alterations and the middle turbinate, is quite variable. Regardless of the technique used, it is vital that the natural drainage ostia are included in surgical dissections to prevent the mucus recirculation phenomena with possible perpetuation, or even worsening, of the chronic condition.
The traditional ESS was the initial proposal focused on the removal of the disease, particularly located in the ostiomeatal complex, with enlargement of the natural drainage ostia and maximum preservation of the sinonasal mucosa. The procedure quickly developed into dissection and more extensive resections, including polypoid tissue, osteitis, and enlargement of other natural drainage ostia, when affected. Another objective of the technique is the possibility of allowing further penetration of topical medications postoperatively. For that purpose, larger openings of the paranasal sinuses are necessary.436 However, some authors choose the surgical approach focused only on the transition spaces of sinuses (pre-ostial spaces of natural sinus drainage through which the air circulates and secretions are drained), regardless of disease extent.516,517 In this technique, called minimally invasive sinus technique (MIST), the drainage ostia are not extended and the procedure is performed mostly with the microdebrider. At the opposite extreme, a technique called nasalization is used, particularly in the surgical management of severe CRSwNP.518 It consists of complete sphenoethmoidectomy with mucosal removal associated with total resection of the middle turbinate.
A major obstacle to this discussion is the lack of well-designed comparative studies to allow for a more standardized behavior among surgeons. More importantly, is the lack of a precise understanding of the etiological factors of CRS, as well as the great variability and disease severity among patients. Thus, the inclusion and exclusion criteria of studies often do not take into account such factors as the associated presence or absence of polyps, greater or lesser degree of eosinophilia, intolerance to anti-inflammatory steroids etc. The great deciding factor for surgeons is the endoscopic and tomographic findings.
For these reasons, current guidelines indicate that surgical treatment should be individualized according to the patient's clinical situation,1 typically correlating greater extent of the disease with a more extensive procedure.
Masterson et al.519 compared anterior ethmoidectomy with complete ethmoidectomy in 149 patients with CRSwNP undergoing ESS. The three-year follow-up showed polyp recurrence in 12.5% of patients submitted to anterior ethmoidectomy versus 4% of those undergoing complete ethmoidectomy. The study also demonstrated that, in experienced hands, the complication rates are small and do not depend on the extent of the procedure.
More recently, Wu et al.520 evaluated the factors affecting the time to revision surgery in patients with CRSwNP. The authors observed that middle turbinate resection appears to increase the time of effectiveness of endoscopic surgery in these cases. This is in agreement with data previously described by Jankovski et al.518
Several studies, mainly case series, have demonstrated very high success rates of ESS, as traditionally described (88%, on average).521–523 However, recent evaluations showed that, given the high degree of mucosal inflammation, surgical outcomes are worse.524–526 Moreover, these patients may have better outcomes with more radical and extensive surgery, such as endoscopic maxillary mega-antrostomy and endoscopic modified Lothrop procedure.527–529 Fortunately, technological improvements allow these more radical techniques to be performed safely by endoscopic endonasal approach in patients with CRS recalcitrant to medical treatment and to traditional endoscopic surgery.
The advent of the microdebrider brought great advances in endonasal surgery. By facilitating the removal of structures safely and quickly, in addition to maintaining the operative field clean by constant aspiration of blood and secretions, the microdebrider is an important assistant, especially in cases of extensive polyposis.530 The use of the neuronavigator, especially in cases of extensive surgery or reoperation cases when there are no reliable anatomical parameters, significantly increased the safety of the procedure.531 Another technique is the use of balloon sinuplasty.511 The procedure involves inserting a catheter with a balloon of different sizes through the transition space of the paranasal sinuses; after verifying its location in the drainage ostium (transillumination or image), it is used to enlarge the ostia, without tissue resection. The procedure is also feasible for the maxillary, frontal, and sphenoid sinuses, but it is not useful for the ethmoid. The procedure is relatively simple to perform and is theoretically compelling, because there is less trauma to the non-affected intranasal structures and a decreased risk of post-operative scarring. However, the few comparative studies with traditional endoscopic techniques have failed to demonstrate convincing results that would justify its high cost.511,532–534
Many authors consider balloons as instruments and not as a surgical technique, much in the same way as they use the microdebrider or neuronavigator and perform hybrid surgeries. That is, together with traditional endoscopic dissection, for instance, of the ethmoid, balloon catheters are used to dilate the natural drainage ostia of the other paranasal sinuses.535
Postoperative topical treatmentSeveral products are available for postoperative topical treatment. They can be applied at high or low volumes with high, low, or negative pressure.536 The capacity of the drug to reach the appropriate anatomical region in the paranasal sinuses has been the subject of extensive research in the past five years. Effective topical therapy depends on several factors, including application technique, postoperative sinonasal anatomy, and fluid dynamics (volume, pressure, position). These combined factors appear to have significant impact on the effectiveness of topical therapy on the affected sinonasal mucosa.537–540
The mechanical removal of mucus, antigens, pollutants, bacteria, and inflammatory products/biofilms is the goal of the topical treatment. These interventions often depend on high-volume and positive-pressure solutions to provide shearing forces that change the surface tension between liquid and air. However, the same approach may not be suitable for the use of pharmaceutical compositions that require properties aimed at complete distribution in the paranasal sinus, long period of contact with the mucosa for local absorption, and minimal waste.536
It is of utmost importance to continue medical treatment postoperatively in almost all forms of CRS. Currently, nasal saline lavage and topical nasal steroids are recommended after ESS for CRS.437,536
The use of medication directly at the disease site has the advantage of allowing high local doses and minimizing side effects.537
The topical solution distribution into the non-operated sinuses appears to be limited. Thus, ESS is essential to allow effective topical distribution to the paranasal sinuses.1 The postoperative distribution is superior with positive-pressure, high-volume devices.538–540 Low-volume sprays and drops show poor distribution, and should be considered only as treatment for the nasal cavity, especially before the ESS. There are limited data on the exact volume necessary to allow complete distribution. Among the topical therapies used in the postoperative period of ESS, the following are noteworthy:
Topical corticosteroidsThere are four randomized, double-blinded, placebo-controlled, level 1b trials that evaluated the use of topical corticosteroids in the early postoperative period; three of them recommend their use,541–543 and only one did not show any benefits.544
CRSsNPThe effectiveness of topical corticosteroids has been investigated in different studies and almost all have shown that CCs can reduce patient symptoms.545 Studies have compared the effectiveness of clinical treatment in patients not submitted to surgery with clinical treatment after ESS, showing that the latter group presented a better response to treatment and better olfactory function, based on objective and subjective criteria.545
In patients with CRSsNP, only those who had undergone prior ESS showed symptom improvement with topical corticosteroid use. When the types of corticosteroids were evaluated, comparing the more recent (mometasone, fluticasone, ciclesonide) with the first-generation drugs (budesonide, betamethasone, triamcinolone, dexamethasone), no significant difference was observed regarding symptoms in either group.1 A recent Cochrane review has demonstrated that the use of topical corticosteroids in patients with CRSsNP showed greater benefit in symptom control when they were introduced directly in the sinus cavity, instead of using common nasal sprays.546
CRSwNPTopical nasal corticosteroids are safe medications to use in postoperative period of patients with CRSwNP, with significant improvement in symptoms, polyp size and rate of polyp recurrence in the first year after the surgery.547
Patients in the postoperative period of sinonasal surgery responded better to topical corticosteroids than those who did not undergo surgery with respect to a decrease in the size of polyps. However, symptom and nasal airflow improvement were not statistically different between the two groups. In a study by Rowe-Jones et al.,541 109 patients (77 of whom had polyps) were randomized to receive fluticasone spray postoperatively, starting six weeks after ESS. The change in the visual analog scale and the endoscopic alterations of polyps were significantly better in the fluticasone group in five years, whereas more courses of prednisolone were prescribed in the placebo group in this period.
There appears to be no significant benefit in using newer corticosteroids (mometasone, fluticasone, and ciclesonide) in comparison to the first-generation drugs (budesonide, beclomethasone, triamcinolone) for symptom or polyp reduction.547
Topical antibioticsThe use of topical antibiotics is controversial, with disagreement about the choice of antibacterial agent to be used, the dosage, method of use, and whether effectiveness is improved in a cavity postoperatively. Studies have been conducted with topical preparations of tobramycin, mupirocin, neomycin, bacitracin/colimycin, and ciprofloxacin. There is insufficient evidence to support a clear benefit of using topical antibiotics in the postoperative period of ESS.437
Nasal irrigation with saline solutionMany theories about the potential physiological benefit of using nasal saline solution irrigation have been proposed, such as improvement in mucus clearance, increased ciliary beat activity, and break-up and removal of antigens, biofilm, and inflammatory mediators, as well as direct sinus mucosal protection. The use of nasal saline irrigation has been recommended by otorhinolaryngologists, both as an adjuvant therapy for chronic sinonasal symptoms, as well as to moisten and cleanse sinonasal clots and crusts and promote mucosal healing in the postoperative period.437
Isotonic or hypertonic saline solutions are often used as nasal shower (irrigation with high-volume and low positive-pressure syringes to achieve a greater degree of mechanical debriding), nasal spray, or nebulizer for the treatment of paranasal sinus diseases, especially as an adjuvant to other therapies, such as ESS.532,548
Nasal saline solution irrigation can improve chronic RS symptoms, especially after sinonasal surgery.
Depending on the mode of application, the penetration of isotonic or hypertonic saline solutions in the paranasal sinuses differs, depending on whether patients are submitted to ESS or not.
Wormald et al.549 compared the use of nasal spray, nasal mist, and nasal showers in patients undergoing ESS. Each method was tested using a technetium tracer. The accumulation of radioactivity was assessed in the anterior and posterior nasal cavity, maxillary, sphenoid, and frontal sinuses, in addition to the frontal recess. Although the nasal cavity was well irrigated by the three techniques, the spray was significantly more effective in penetrating the maxillary sinus and frontal recess compared to the other methods. The sphenoid and frontal sinuses were poorly irrigated by all three methods.
The nasal shower effectiveness after surgery was evaluated in a blinded RCT. Nasal showers were used by 22 patients after surgery on one side of the nasal cavity, three times a day for six weeks. The opposite nasal cavity was not irrigated. The presence of adhesions, polyps, crusts, secretions, or edema was evaluated three weeks and three months after surgery. At three weeks, the saline solution showers improved the presence of secretion or edema, but had no effect on adhesion or crusts. At three months, there were no significant differences between both nasal cavities.550
A study that evaluated three randomized trials in patients after sinonasal endoscopic surgery demonstrated that two of them obtained better outcomes with intranasal saline solution when compared with the group without irrigation, whereas the third showed no difference in symptom scores between patients who used hypertonic saline solution and those without irrigation. Patients who used hypertonic saline solution had more pain. In general, normal saline solutions are well tolerated. Side effects, including nasal discomfort, nasal discharge, epistaxis, headache, and earache are rare. Most studies show improvement in symptoms and quality of life with the use of nasal irrigation, but whether the hypertonic saline solution is superior to the isotonic solution remains unclear.551
Rudmik et al.552 reviewed six studies on care after ESS and found that most of them showed improvement in symptom scores using saline solutions postoperatively. Among these studies, the one by Liang et al.553 compared irrigations with saline solutions combined with postoperative debridement versus debridement alone, and found that postoperative debridement combined with the use of saline solution irrigation significantly improved the endoscopic appearance and symptoms in patients with mild CRS, although no improvement was observed in moderate and severe cases. The authors concluded that nasal irrigation with saline solution is well tolerated and improves the endoscopic appearance and early postoperative symptoms, with evidence level 1b and 2b.552 Nasal lavages are therefore recommended for CRS in the postoperative period in adults.
Others- •
Surfactants: There are no RCTs to recommend the use of surfactants (including baby shampoo) postoperatively.554,555
- •
Antifungals: No benefit was observed in RCT or systematic reviews on the use of topical antifungals in the postoperative period of CRS.437,486
- •
Furosemide: Based on current data of long-term postoperative period of nasal surgery, treatment with furosemide is not recommended.556
- •
Capsaicin: RCT was performed in patients at the postoperative period of ESS using capsaicin (cotton swab) into the middle meatus of both nostrils for 20minutes once a week for five weeks, comparing them with the control group. The treated group showed improved staging of polyposis; this is a grade C recommendation.1,556
Nasal lavage with isotonic saline solution may be used in the immediate postoperative period of CRS, as well as topical nasal corticosteroids, which can be initiated two to three weeks after surgery, or after the disappearance of crusts. There are no relevant data in the literature to support the use of other topical nasal agents in the postoperative period of CRS.
Postoperative systemic treatmentPostoperative systemic treatment of CRS with or without nasal polyps may involve the use of corticosteroids and antibiotics, and is discussed below.
CorticosteroidsAfter the surgical treatment of CRS, systemic corticosteroids may be used in mainly two ways: in short doses, between seven and 14 days, with a maintenance dose throughout treatment, or for longer periods, using decreasing doses.556,557 The main role of corticosteroids in this type of disease is to reduce mucosal inflammation, thus providing better surgical results. However, these drugs are still avoided by many surgeons due to their potential side effects.
A phase 1b trial (randomized, double-blinded, placebo-controlled), performed by Wright and Agrawal,507 evaluated the endoscopic findings in the postoperative period in patients with CRSwNP, who used 30mg daily of prednisone five days before the surgical procedure, plus 30mg a day for nine days postoperatively. In addition to a better intraoperative status in the patients taking corticosteroids, the results showed significant improvement in endoscopic mucosal appearance up to six months postoperatively, more evident in the second week. It is worth mentioning that, regarding postoperative symptoms, there was no difference between the treatment groups. Both groups (placebo and prednisone) showed significant improvement postoperatively when compared with the preoperative period.
After careful assessment of the risks and benefits, the use of oral corticosteroids for short periods of time can be considered, aiming to minimize inflammation during the healing period and prevent complications associated with mucosal edema and crust formation, especially in cases of CRSwNP.556
Regarding the use of corticosteroids in the postoperative period of patients with AFRS, many non-placebo-controlled studies showed a positive effect.558–560 Rupa et al.,561 in a prospective, randomized, double-blinded, placebo-controlled trial (level 1b), compared the outcomes of patients submitted to surgery with a diagnosis of AFRS. One group of patients received 50mg of prednisone orally a day for six weeks and subsequently, decreased doses for six weeks, while another group was given placebo for 12 weeks. After this period, a significant improvement was observed regarding symptoms and at the endoscopic examination in the group that had used corticosteroids. All patients received topical corticosteroids and systemic antifungal (itraconazole) for 12 weeks. At 18 months postoperatively, patients who had interrupted the treatment, including topical corticosteroids, had disease recurrence. It is therefore difficult to assess whether treatment with oral corticosteroids for 12 weeks had an impact on evolution at 18 months.
AntibioticsThe purpose of antibiotic use postoperatively is to prevent infection of the secretions retained in the paranasal sinuses immediately after surgery. If there is purulent secretion during the surgical procedure, antibiotics should be prescribed, based on the culture and sensitivity test. Otherwise, antibiotics effective against the most common pathogens should be employed.557 A meta-analysis and systematic review published in 2011, that included three articles, demonstrated that the prophylactic use of antibiotics in the postoperative period did not result in statistically significant reduction of infection, endoscopic scores, and symptoms.562 It is worth mentioning the randomized, double-blinded, placebo-controlled trial (level 1b) conducted by Albu et al.,563 which evaluated the protocol for the use of 625mg of amoxicillin twice daily for two weeks postoperatively. The results showed improvement of symptoms within the first five days and of the endoscopic appearance within the first 12 days. Additionally, there was a significant reduction in crust formation. Another level 1b study assessed the use of antibiotics for just two days postoperatively and observed no effective result.564
There is only one level 1b study, published in 1995, which found benefits regarding the use of macrolides for long periods (12 weeks) postoperatively.565 As there are no other specific studies that assessed the effects of this medication specifically in the postoperative period, macrolides have been used for a long time, regardless of the postoperative period. In this sense, the evidence is contradictory; in light of current knowledge, it points to possible positive results in patients with CRSsNP and normal IgE.426,427
CommentsIn spite of the scarcity of literature data on antibiotic effectiveness in the postoperative period of endoscopic sinus surgery, it is believed that they can improve symptoms and endoscopic appearance, if used for a longer period (at least 14 days), but there is no conclusive data about the duration of these benefits. In general, penicillin derivatives, particularly amoxicillin-clavulanic acid and cefuroxime-axetil are the most commonly used.
AntifungalsKennedy et al.207 performed a single controlled trial (1b) comparing the use of the antifungal terbinafine with placebo in patients with chronic RS with nasal polyps who showed (or did not show) positivity for fungi. The results demonstrated that terbinafine did not improve symptoms or post-operative radiological findings even in cases where the culture was positive for fungi. A number of other non-controlled trials showed conflicting results regarding the postoperative use of systemic antifungals in CRS, with some of them showing significant side effects of such medication.492,493,561,566 Based on current data, the use of systemic antifungals in the postoperative period of chronic RS with nasal polyps is not recommended.1
Special aspects of rhinosinusitis in childrenEpidemiologyOn average, children younger than 5 years of age have between two and seven episodes of upper respiratory tract infections (URTI) per year.567,568 If they attend kindergarten and daycare centers, the number episodes increases to 14 per year.569 It is estimated that 4% to 7.3% of URTIs develop into ABRS, occurring most often in children in their first year of life and those attending daycare.570
Paranasal sinuses in the childNot all paranasal sinuses are developed at birth.
- •
Frontal sinus: its development starts at 4 years of age, with slow growth thereafter. Only 20% to 30% of children younger than 6 years have a radiographically visible frontal sinus. Over 85% will have frontal sinus pneumatization on CT by the age of 12.571
- •
Ethmoid and maxillary sinuses: they are already developed enough at birth; these are the ones that have clinical significance in RS. The ethmoid sinuses grow rapidly until 7 years of age, and their development is complete at around 15-16 years of age. The maxillary sinus usually is pneumatized at birth and its volume is approximately 2mL at 2 years of age, 10mL at 9 years, and 14.8mL at 15 years.572 Most of the growth after 12 years of age occurs in the lower portion, with pneumatization of the alveolar process occurring after the secondary dentition. The floor of the maxillary sinus, which is higher than the nasal cavity floor level in children, will lower, and it will be approximately 4-5mm inferior to the nasal cavity in adult life.
- •
Sphenoid sinus: at birth, the sphenoid sinus is a small evagination of the sphenoethmoidal recess. At age 7 years, it extends posteriorly, and at age 8, around 85% of patients have pneumatization that is visible on CT;571 it reaches full development at approximately 15 years, but it can continue to grow into adulthood.
ARS is defined and classified in children in the same way as it is in adults.
DiagnosisThe clinical diagnosis of ARS in children is not easy to establish. Many symptoms are common to other childhood diseases such as colds, flu, and allergic rhinitis. Additionally, there are limitations and difficulties in the clinical examination of the pediatric population.
Most frequent signs and symptomsStudies in children with ARS show that the clinical picture often includes fever (50% to 60%), rhinorrhea (71% to 80%), cough (50% to 80%), and pain (29% to 33%),38 in addition to retronasal secretion and nasal obstruction.19 In children up to preschool age, the pain symptom has low prevalence, being replaced by cough, whereas in schoolchildren and adolescents, pain becomes more common.
Although there are not many studies on the subject, most physicians and guidelines recommend that the diagnosis of ABRS should be clinical, based on time of evolution (URTI symptoms lasting more than ten days), abrupt onset of marked symptoms (as early as in the first four days), or symptoms worsening after the initial period of improvement during a URTI, known as “double worsening”. High fever, abundant nasal purulent discharge, periorbital edema, and facial pain may be part of the signs and symptoms.1,19,573–576
Clinical examinationIn addition to the signs and symptoms mentioned above, nasal endoscopy helps in the diagnosis and differentiates between viral and bacterial cases by allowing visualization of nasal and nasopharynx secretions. When it identifies purulent secretion draining from the middle meatus it establishes the diagnosis for acute bacterial RS. However, it is not always easy to perform in children. Moreover, despite the high specificity, it has a low degree of sensitivity, as a negative examination does not exclude the diagnosis of ABRS.
Imaging studyThere is practically a consensus, among all the most recently published guidelines, that the diagnosis of ARS should not be based on radiological examinations, particularly plain radiographs.1,573,576
Viral processes in children often involve the paranasal sinuses. Children with symptoms of URTI for at least six days of clinical picture usually show signs of abnormalities in all sinuses: maxillary and ethmoid, sphenoid, and frontal, in order of frequency. The opacification is nonspecific and may occur in viral, bacterial, allergic processes, as well as in tumors, or simply reflect a lack of sinus development.
CT studies in children with a clinical picture suggestive of ARS demonstrated that even the most important clinical pictures show significant alteration regression after two weeks.577 Indications for CT in acute sinus pictures should, therefore, be reserved for patients who do not improve and whose symptoms persist after appropriate therapy, as well as in those with suspected complications.574
Differential diagnosisThe main differential diagnosis of ARS in children is infectious acute adenoiditis, as it might show very similar signs and symptoms, including cough and posterior secretion. There is probably a high percentage of association between the two diseases, although this differentiation is difficult to attain in clinical practice. Studies demonstrated that, of children with symptoms for over ten days, approximately 89.2% have ARS, whereas 19.2% have associated adenoiditis. Adenoiditis alone is present in approximately 7% of the children. Younger patients (2 to 5 years old) have a higher frequency of ARS/adenoiditis association.578 In clinical practice, differential diagnosis is not always necessary, as treatment is the same for both entities.
Another less important differential diagnosis is that of nasal foreign body. In these cases, the secretion is usually fetid and almost always unilateral.
BacteriologyThe most common etiologic agents in ABRS in children are S. pneumoniae, H. influenza, M. catarrhalis, S. pyogenes, and anaerobic bacteria.19,579
ARS drug treatment in childrenMost are self-limited, resolving spontaneously.1
AntibioticsThe results of a meta-analysis suggest that the rate of resolution and improvement in ARS between seven and 15 days is slightly higher when antibiotics are used.579 For this reason, it is believed that antibiotics should be reserved for more severe cases or in the presence of concomitant diseases, which could be exacerbated by ARS, such as asthma and chronic bronchitis.1,573,575 However, there is yet no universal consensus on the type of antibiotics to be used in ARS.
In general, amoxicillin (40mg/kg/day or 80mg/kg/day) is still considered a sensible initial treatment in most studies. Amoxicillin/clavulanate and cephalosporins are considered good options against beta-lactamase producers,1 and must be indicated in case of a first treatment failure.
Similar to the recommendations for acute otitis media, in ARS there is also the option of a single dose of ceftriaxone 50mg/kg IV (intravenous) or IM (intramuscular) for children who are vomiting, unable to tolerate oral medication.62–64 If there is clinical improvement in 24hours, treatment is completed with an oral antibiotic.575
For patients allergic to penicillin, there is some controversy among the latest international guidelines. Some consider trimethoprim-sulfamethoxazole, macrolides, and clindamycin to be good options1 in these situations. Others do not recommend the use of trimethoprim-sulfamethoxazole and macrolides due to the increasing resistance of pneumococci and H. influenzae to these drugs, suggesting a quinolone, such as levofloxacin, as an alternative, especially in older children, even considering toxicity, cost, and emerging resistance.580,581
There are no reviews on the optimal treatment duration. Recommendations based on clinical observations have varied widely, from 10 to 28 days of treatment. One suggestion has been to maintain therapy for seven days after symptom resolution.582
Intranasal corticosteroidsIntranasal corticosteroids for three weeks combined with antibiotic therapy appears to have advantages in relation to ARS treatment in children and adolescents compared to antibiotic use alone, especially regarding cough and nasal secretion.85,92,95
A single double-blinded, randomized trial in patients older than 12 years found that a double dose of intranasal corticosteroids, as the sole drug used, was more effective for ARS control than treatment with antibiotics alone.85
Adjuvant therapyA systematic review of the literature regarding the efficacy of oral or intranasal decongestants, antihistamines, and saline irrigation has shown no evidence of efficacy in children with ARS.100
Recurrent ARS (rARS)Most authors agree that rARS is defined by acute episodes lasting less than 30 days, with intervals of at least ten days when the patient is totally asymptomatic. According to some authors, the patient should have at least four episodes a year to meet the criteria of recurrence.575
As in chronic conditions, systemic causes should be sought and ruled out. The investigation should include allergic processes, by performing specific tests; immunoglobulin deficiencies, with quantitative determinations, particularly for IgA and IgG; CF; gastroesophageal reflux; and ciliary diseases.583 Pharyngeal tonsil hypertrophy, even when mild, should also be considered because of the possibility of the tonsils acting as a reservoir for pathogens. Although of little relevance in children, anatomical factors, such as concha bullosa and septal deviation should also be ruled out. In these cases, CT, nasal endoscopy and/or MRI can assist in the diagnosis of obstructive processes and malformations.
The bacteriology is the same of ARS and, therefore, the treatment of the acute phase should follow the same principles.584 Unfortunately, it must be acknowledged that several antibiotic agents taken in a short intervals of time can lead to bacterial resistance. Prophylaxis with antimicrobials should be reserved for exceptional cases, usually when underlying diseases are confirmed particularly immunodeficiencies.
Annual vaccination for influenza and pneumococcal vaccine are recommended as general prophylactic measures. In cases where allergic rhinitis or gastroesophageal reflux is present at the same time, the frequency of acute events decreases when the associated disease is treated. Several studies have demonstrated that immunostimulatory medications, such as bacterial lysates, help to control recurrent viral and bacterial RTIs, and may be an adjunct treatment in rARS control.113
Particularities of chronic rhinosinusitis in childrenCRS is not as frequently studied in children as it is in adults, and its prevalence has not yet been fully established. It is believed that several factors contribute to the disease, including inflammatory and bacteriological components, and that the pharyngeal tonsil is an important consideration in this age group. Treatment is mainly medical, and surgical therapy is reserved for a minority of patients.
DefinitionCRS is defined in children, similarly to adults, as inflammation of the nasal mucosa and paranasal sinuses having a duration equal to or longer than 12 weeks without improvement periods.1
PhysiopathologyAnatomical factorsIt is unclear whether the anatomical abnormalities somehow contribute to the development and maintenance of CRS in children. Studies suggest that, in spite of the common occurrence of these anatomical factors (concha bullosa, concha hypertrophy, septal deviation, among others), they do not appear to be correlated with the presence of CRS or the degree of involvement.1
Role of adenoidsStudies related to the role of adenoids in CRS are being conducted, but are still limited. They suggest a role for adenoids in patients with CRS, both from the bacteriological and immunological viewpoints. All of them confirmed the hypothesis that, regardless of the size of the pharyngeal tonsil, they can be a reservoir for bacterial sinus infections.585,586
Allergic rhinitisApparently, children with CRS have positive radioallergosorbent tests (RAST) for IgE, in the same proportion as the general population, suggesting that a causal relationship between CRS and allergies in children remains controversial and probably does not exist.587,588
AsthmaAsthma is a disease commonly associated with CRS in pediatric patients. However, the limitations of most available studies include lack of good controls or randomization to different treatment modalities and, therefore, the association between CRS and asthma in children should be further studied.
GERDGERD has also been associated with RS in several studies. Despite some evidence demonstrating an association between GERD and CRS, more controlled trials are needed to reinforce this association and validate it. Thus, routine antireflux treatment of children with CRS is not justified.1
ImmunodeficiencyStudies in children with rARS (recurrent acute rhinosinusitis) and CRS have shown reasonable percentage of immune dysfunction, including decreased levels of IgG3, IgA, and IgG1, with poor response to pneumococcal vaccine and low levels of immunoglobulin in response to normal vaccines. Therefore, it is recommended to evaluate immune function in children with recurrent CRS through immunoglobulin measurements and titrations of tetanus and diphtheria, as well as of pneumococcus. If responses are abnormal, examinations should be repeated after pneumococcal vaccination.1
PCDThe diagnosis should be suspected in a child with atypical asthma, bronchiectasis, chronic productive cough, CRS, and severe otitis media (especially chronic drainage in children with tubes). Specific diagnosis requires examination of cilia by electron microscopy, which is usually available in specialized centers.1
CFThe prevalence of CRS in these patients is high and nasal polyps occur between 7% and 50% of affected individuals.589,590 This is one of the few causes of NP in children and the finding is unusual; when present, it should lead to a suspicion of CF or AFRS. This also shows a fairly unique clinical picture, which includes NP and characteristic CT and MRI images.591
Clinical picture and diagnosisThe clinical diagnosis of chronic RS in children is still considered a challenge, as it often overlaps with those of other common childhood illnesses, such as viral infections of the upper respiratory tract, hypertrophy of the pharyngeal tonsils/adenoiditis, and allergic rhinitis. The most important signs and symptoms include nasal obstruction/stuffiness/congestion, rhinorrhea (anterior/posterior); less commonly facial pain/ pressure, and cough. CT and endoscopic examination can show relevant changes in the nose, paranasal sinuses and mucosa.1
Imaging testsStudies that have assessed the incidence of abnormalities in the paranasal sinuses on CT obtained for clinical reasons unrelated to the CRS in children have shown a percentage of radiographic sinus abnormalities ranging from 18%2,3 to 45%, similar to those found in children with CRS symptoms. This demonstrates that the significance of imaging tests is relative and must always be considered together with the clinical picture.
BacteriologyThere are few studies on the bacteriology of CRS in children. Microorganisms identified intraoperatively or in aspirates include: S. alpha hemolytic, S. aureus, S. pneumoniae, H. influenzae, and M. catarrhalis, as well as anaerobic organisms such as bacteroides and Brook I fusobacteria.592–594
TreatmentDrug therapyExisting studies demonstrate that short-term antibiotic therapy in children with CRS is not justifiable.1 Conversely, both nasal corticosteroids and saline solution have shown to be beneficial and are considered the first-line treatment for this disease, whether with or without nasal polyps.595,596
Surgical treatmentThe surgical approach should always be reserved for special cases, i.e., children who have not responded to appropriate medical treatment. Studies have shown significant clinical and quality of life improvement, without negative effects in relation to facial osteoskeletal sequelae.597 Unfortunately, most studies supporting this recommendation are not prospective and randomized. In general, the surgical approach, when indicated, may consist first of an adenoidectomy597 with maxillary sinus lavage.598 The surgery can be performed with or without balloon dilation,599,600 followed by endoscopic paranasal sinus surgery in cases of recurrence of symptoms.601 In cases of children with CF, NP, antrochoanal polyps or AFRS, endoscopic surgery is the first option. Perhaps future studies, comparing the several methods of treatment with standardized preand postoperative questionnaire, can guide the best therapeutic approach in patients with CRS.




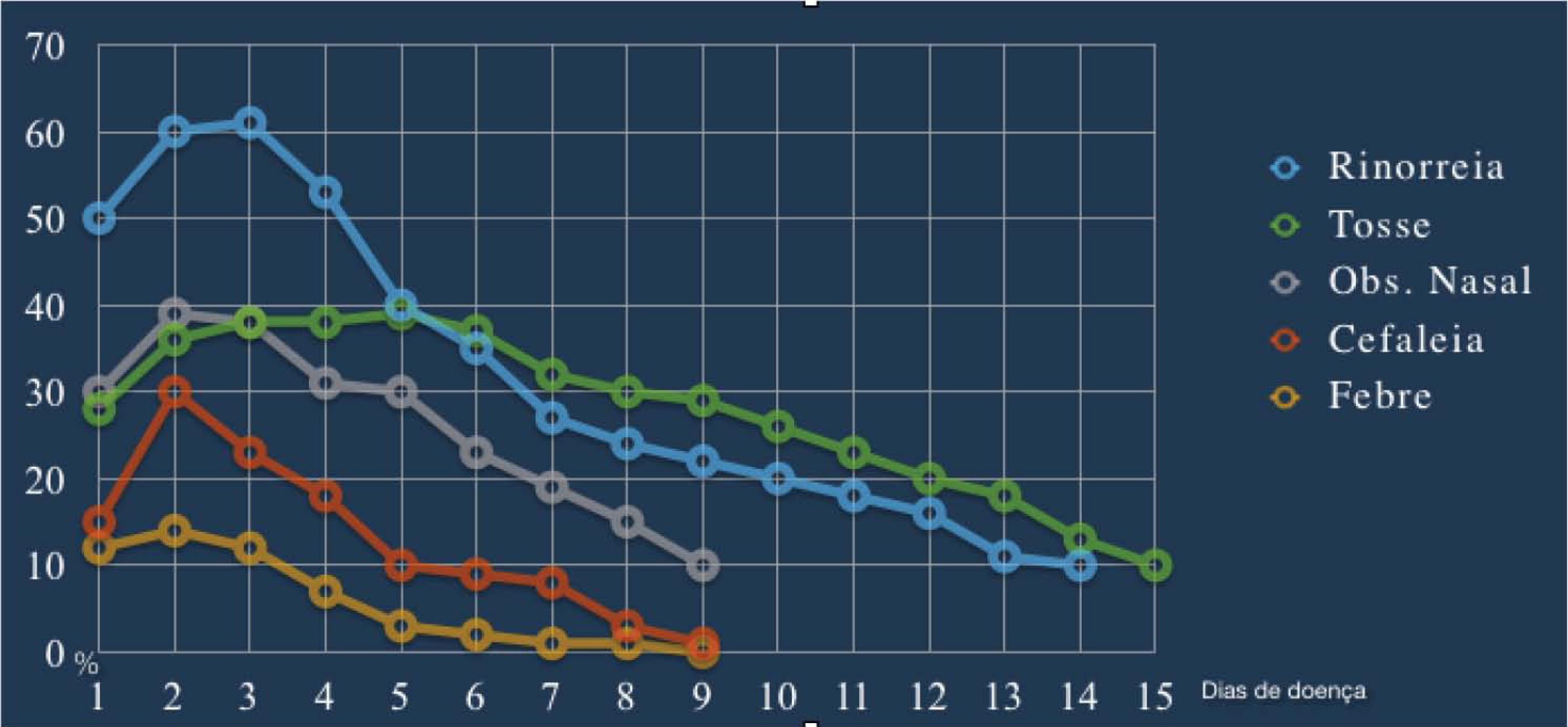
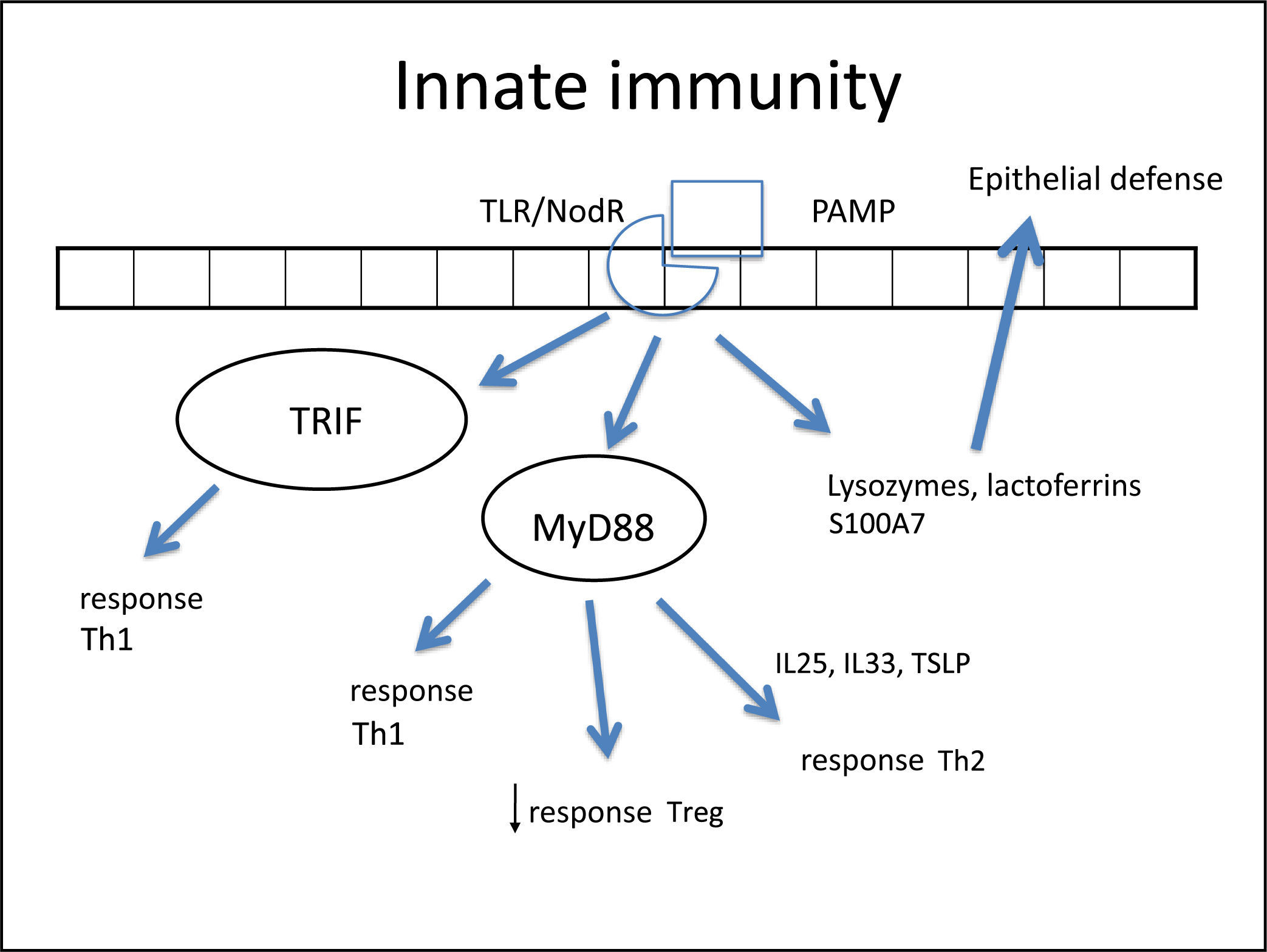
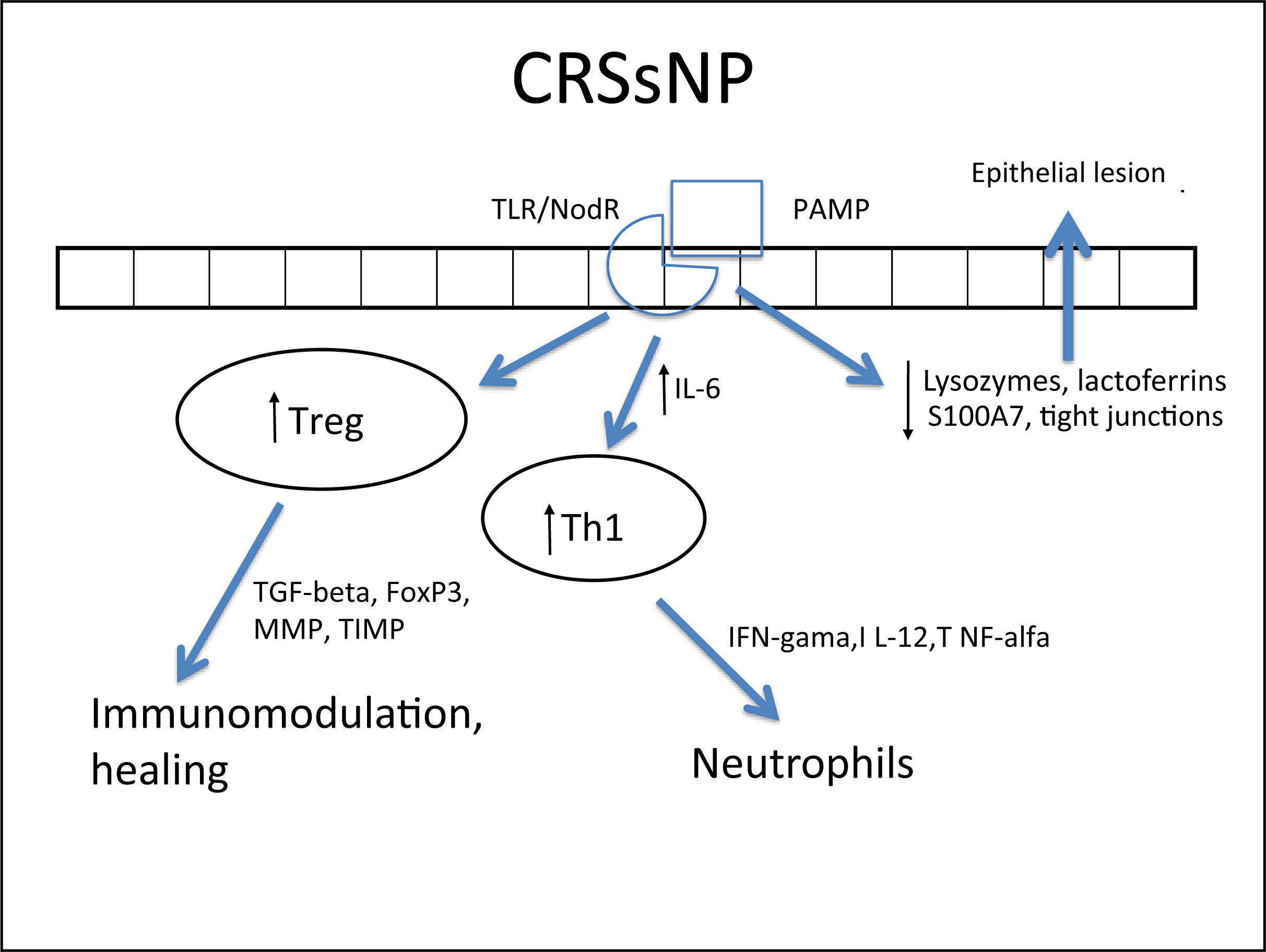
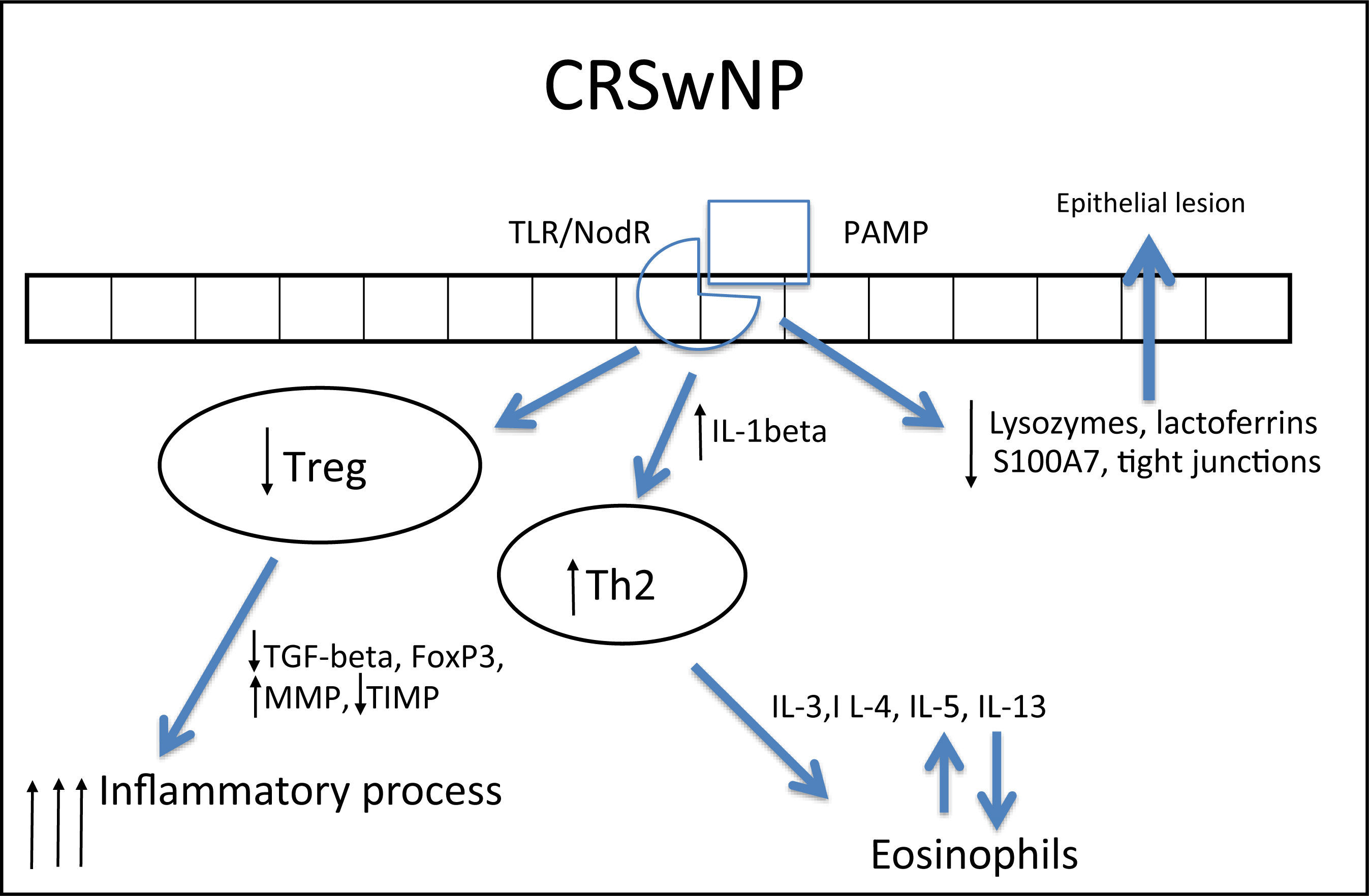

![Rhinosinusitis symptoms of acute infection caused by rhinovirus in relation to the start time and duration. (Adapted from Gwaltney et al. [1967]).59 Rhinosinusitis symptoms of acute infection caused by rhinovirus in relation to the start time and duration. (Adapted from Gwaltney et al. [1967]).59](https://static.elsevier.es/multimedia/18088694/00000081000000S1/v1_201601280921/S1808869415000051/v1_201601280921/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/7hLX2FbBoxC1192i158SI=)