Several methods have been proposed to improve operative success in renal hyperparathyroidism.
ObjectiveTo evaluate stereomicroscopy in parathyroid tissue selection for total parathyroidectomy with autotransplantation in secondary (SHPT)/tertiary (THPT) hyperparathyroidism.
Methods118 renal patients underwent surgery from April of 2000 to October 2009. They were divided into two groups: G1, 66 patients operated from April of 2000 to May of 2005, with tissue selection based on macroscopic observation; G2, 52 patients operated from March of 2008 to October 2009 with stereomicroscopy for tissue selection searching for the presence of adipose cells. All surgeries were performed by the same surgeon. Patients presented SHPT (dialysis treatment) or THPT (renal-grafted). Follow-up was 12–36 months. Intra-operative parathyroid hormone (PTH) was measured in 100/118 (84.7%) patients.
ResultsData are presented as means. G1 included 66 patients (38 SHPT, 24 females/14 males; 40.0 years of age; 28 THPT, 14 females/14 males; 44 years of age). G2 included 52 patients (29 SHPT, 11 females/18 males; 50.7 years of age; 23 THPT, 13 females/10 males, 44.4 years of age). SHPT patients from G2 presented preoperative serum calcium higher than those of SHPT patients in G1 (p<0.05), suggesting a more severe disease. Definitive hypoparathyroidism was found in seven of 118 patients (5.9%). Graft-dependent recurrence occurred in four patients, two in each group. All occurred in dialysis patients.
ConclusionStereomicroscopy in SHPT/THPT surgical treatment may be a useful tool to standardize parathyroid tissue selection.
Diversos métodos têm sido propostos com intuito de melhorar índices de sucesso cirúrgico no tratamento do hiperparatiroidismo associado à doença renal crônica (DRC).
ObjetivosAvaliar uso do estereomicroscópio na seleção de tecido paratiroideano na paratiroidectomia total com autoimplante em pacientes com DRC.
Métodos118 pacientes DRC operados entre 04/2000-10/2009 foram divididos em: G1-66 pacientes operados entre 04/2000-05/2005 cuja seleção de tecido foi realizada por método convencional (macroscopia); G2-52 pacientes operados entre 03/2008-10/2009, cuja seleção de tecido foi realizada com uso da estereomicroscopia: Leica-Stereomicroscope (amplificação: 10×-80×). Pacientes foram ainda categorizados em hiperparatiroidismo secundário (HPS) ou terciário (HPT) (HPS-diálise/HPT-transplantados renais). Seguimento pós-operatório: 12-36 meses. PTH intraoperatório medido 100/118 pacientes (84.7%). Todos pacientes foram operados pelo mesmo cirurgião.
ResultadosDados em média. G1, 66 pacientes (38 HPS, 24f/14m; 40 anos; 28 HPT, 14f/14m; 44 anos). G2, 52 pacientes (29 HPS, 11f/18m; 50,7 anos; 23 HPT, 13f/10m; 44,4 anos). Pacientes dialíticos do G2 apresentaram cálcio pré-operatório maior que G1 (p<0,05), sugerindo doença mais severa. Hipoparatiroidismo definitivo: 7/118 (5,9%) pacientes: G1, 4/66 (6%); G2, 3/52 (5,7%). Recorrência do hiperparatiroidismo no autoimplante: 4 pacientes, 2 em cada grupo. Todas foram em pacientes em diálise.
ConclusãoEstereomicroscopia no tratamento do hiperparatiroidismo associado à DRC é útil na padronização da técnica de seleção de tecido para o autoimplante.
The longer survival of chronic renal patients has increased the incidence of symptomatic hyperparathyroidism, increasing the need of surgical procedure.1 The best surgical approach for renal hyperparathyroidism is still debated and controversy remains. Relevant questions have been raised regarding treatment election, since post-surgical recurrence and the risk of definitive hypoparathyroidism should be avoided. Total parathyroidectomy with parathyroid tissue autotransplantation is a well-accepted technique for the management of these patients.2–7 Since recurrence rates of 8%8 to 20%9 and up to 76%10 have been reported in the literature, the great majority graft-dependent, tissue selection for autotransplantation has been presented as a considerable challenge. Analysis of parathyroid hyperplastic tumors, using X-chromosome inactivation, have demonstrated that monoclonal transformation is a frequent occurrence in uremic patients.11 In fact, the great majority (64%) of parathyroid glands with generalized hyperplasia (non-nodular component) were unequivocally monoclonal, as demonstrated by Arnold et al.11 This finding imposes a difficult condition in parathyroid tissue selection for autotransplantation.
Intraoperative tissue selection of parathyroid fragments using a stereomagnifier was first described by Neyer et al. in 2002.12 The technique can differentiate parathyroid normotropic areas by the presence of stromal fat cells from those that are disfunctional and hyperplastic tissue without fat cells.12 Cells from these lipid-containing areas present an optimal in vitro suppression of parathyroid hormone (PTH) secretion by high calcium levels,13 indicating a normal calcium set-point and suggesting the eligibility for autotransplantation.
The aim of this study was to compare stereomicroscopy to conventional macroscopic observation in parathyroid tissue selection using a historical control, in order to provide a standardized procedure for renal hyperparathyroidism surgery.
Patients and methodsPatients118 renal patients underwent total parathyroidectomy with intramuscular presternal autotransplantation7 from April of 2000 to October of 2009. Patients were followed-up at the Renal Osteodystrophy Unit in this institution and were referred to surgical treatment for persistent hypercalcemia not responsive to medical interventions and/or persistent hyperphosphatemia despite the continued use of dietary phosphorus restriction and phosphate-binding agents, with signs and symptoms such as intractable pruritus, severe bone pain, fractures or high risk of fracture, skeletal deformities, extra skeletal calcifications, development of calciphylaxis, and radiographic evidences of renal osteodystrophy.
Patients were divided into two groups: group 1 (G1) consisted of 66 patients who underwent surgery from April of 2000 to May of 2005, and parathyroid tissue selection for autotransplantation was performed using conventional technique based on macroscopic parathyroid tissue observation by a single experienced surgeon; group 2 (G2) consisted of 52 patients who underwent surgery from March of 2008 to October of 2009, and parathyroid tissue selection for autotransplantation was performed by the same surgeon as G1, based on stereomicroscopy observation using a Leica Stereo Zoom S8 APO Stereomicroscope (Leica Microsystems GmbH – Wetzlar, Germany), with magnification of 10–80×.
Patients were classified as secondary or tertiary hyperparathyroidism: secondary hyperparathyroidism (SHPT) was characterized as an acquired disorder observed in end-stage renal disease, in which the uremic state presents a continuous stimulus to the parathyroid glands. The SHPT group included patients under dialysis treatment who presented severe hyperparathyroidism with normal or high serum calcium levels. The tertiary hyperparathyroidism (THPT) group comprised renal patients with functioning kidney transplant and nonsuppressible parathyroid hyperplasia, with persistent increased PTH levels and hypercalcemia. Hypercalcemia after kidney transplantation is usually due to hyperparathyroidism that persists from the preceding chronic kidney disease period.14
Surgical cure was defined as restoration of serum calcium and PTH levels14 throughout the first six months after surgery. Recurrence was defined when high levels of PTH were observed throughout late post-operative follow-up (one year after surgical procedure) that failed to respond to medical/pharmacological management. Definitive hypoparathyroidism was defined when PTH measurements under 10pg/mL endured one year after parathyroidectomy, with normal or low serum calcium levels under vitamin D and oral calcium supplementation.
Serum ionized calcium (iCa), phosphorus (P), alkaline phosphatase (AP), and intact parathyroid hormone (iPTH) were measured before parathyroidectomy and every six months after surgery in all patients from both groups.
Regarding the follow-up, patients from both groups were followed-up after surgery on a regular basis in the Renal Osteodystrophy Unit, and patient data from medical reports were available for this study. Patients from G1 underwent surgery from 2000 to 2005, while patients from G2 underwent surgery from 2008 to 2009. Thus, for surgical outcome analysis, the first 36 months were the period selected for study in both groups, since it was the longest period available for G2 patients.
MethodsStudy designThis was a comparative study using a historical control, evaluating stereomicroscopy in comparison to conventional macroscopic technique in parathyroid tissue selection in surgical treatment of renal hyperparathyroidism.
This investigation was approved by the institutional ethics committee (approval No. CEP 0234/06) and patients signed an informed consent prior to their inclusion in the study.
Surgical strategyBilateral cervical exploration with at least four-gland excision confirmed by frozen section examination and/or intra-operative PTH (IO-PTH) measurement was performed in all 118 patients (IO-PTH available in 100 patients).
Removed parathyroid glands were carefully examined in order to select a parathyroid area (non-nodular region) for implant and cryopreservation. The selected parathyroid fragment was gently diced into small pieces measuring approximately 2.0mm3. Approximately 30 parathyroid fragments were implanted in presternal musculature over a single area of 1.5cm in length.7 Another 30 fragments were selected for cryopreservation. The reasons for changing the grafted area from the forearm to the presternal musculature are related to some potential advantages. First, it preserves the forearm for arterio-venousus-fistula dialysis if needed. Second, the presternal region is close to the cervicotomy, exposing the same surgical area. Third, it allows for an easier graft-removal in case of graft-dependent recurrence; the sternal bone presents a posterior boundary for the grafted tissue, enabling a graft removal under local anestesia.7 All surgeries were performed by the same surgeon in both groups G1 and G2.
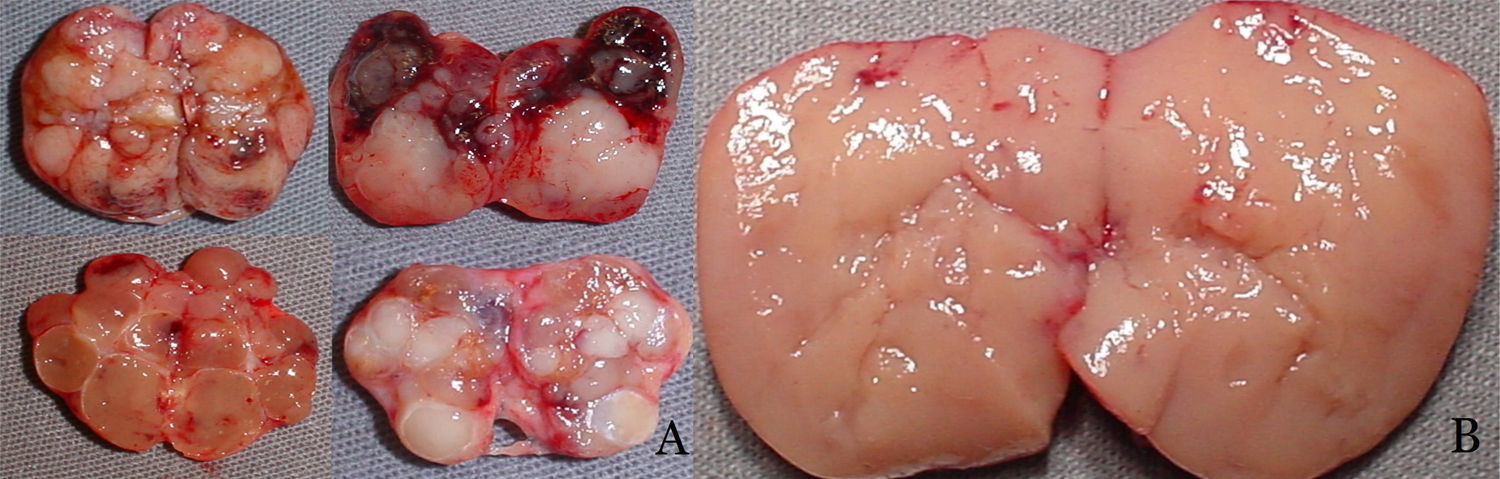
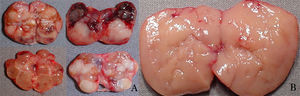
Protocol for intraoperative tissue selectionGroup 1 – Conventional technique: tissue selection for autotransplantation based on macroscopic parathyroid observation was performed in 66 patients. Nodular parathyroid regions were avoided, while hyperplasic non-nodular parathyroid areas were considered eligible for autotransplantation (Fig. 1).
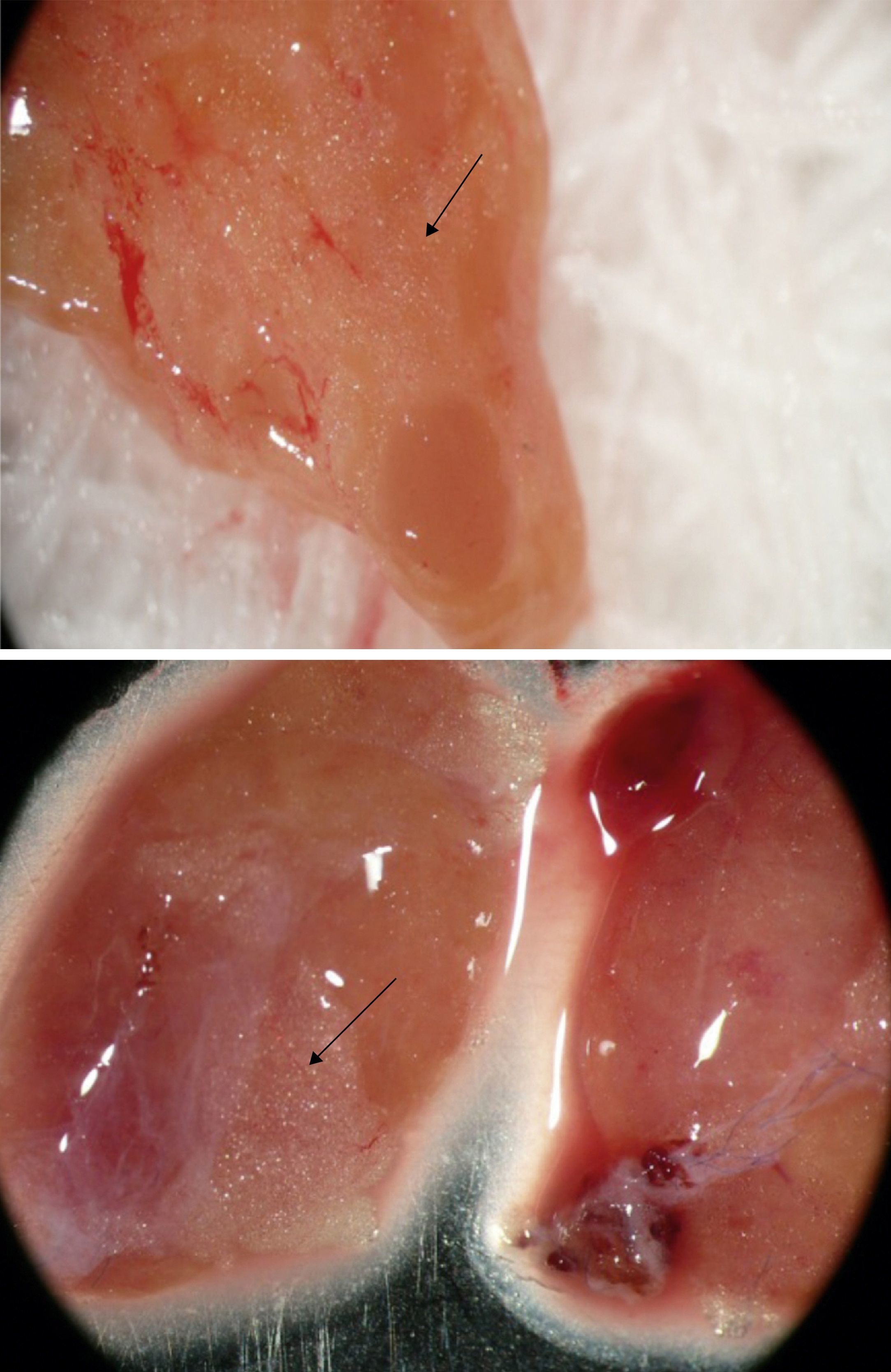
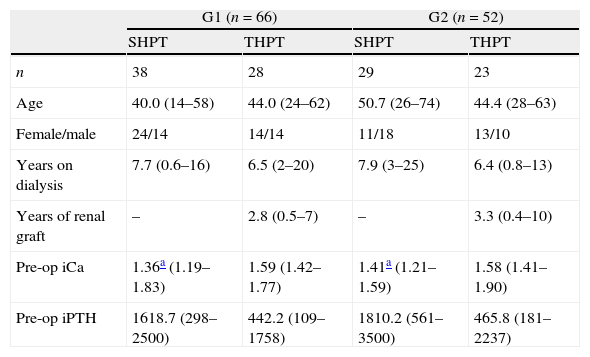
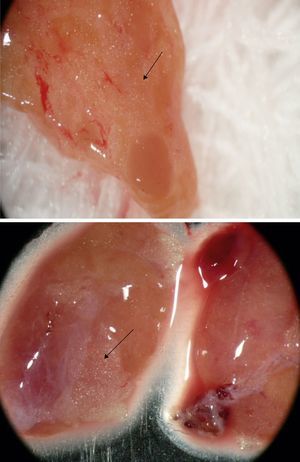
Group 2 – Stereomicroscopy: parathyroid tissue selection using Leica Stereo Zoom S8 APO Stereomicroscope (Leica Microsystems GmbH – Wetzlar, Germany) with apochromatic 8:1 zoom, 10–80× magnification, was performed in 52 patients. Resected parathyroid glands were first observed macroscopically, and stereomicroscopic analysis was performed thereafter, searching for the presence of stromal fat cells in the parathyroid glands (Fig. 2, arrows).
BiochemistryIO-PTH was performed to confirm total removal of the parathyroid glands, in order to avoid overlooking remaining or supernumerary hyperfunctioning glands. IO-PTH was measured using the Elecsys PTH Immunoassay (Elecsys 1010 System; Roche – Mannheim, Germany) and was available for 100 patients (84.7%). The time required to perform the assay is nine minutes and reference values are 10–70pg/mL. Peripheral venous blood sample (4.0mL) was obtained immediately after induction of anesthesia and 20min after removal of all parathyroid glands.15–17
Total serum calcium, phosphorus, total alkaline phosphatase, and creatinine were measured by means of standard automatic assays (Hitachi 912 – Roche). Serum ionized calcium was measured by using an ion-specific electrode (AVL 9180 Electrolyte Analyzer – Roswell, Georgia, United States). Parathyroid hormone in ambulatory conditions was measured by immunometric assay (Immulite; Siemens – São Paulo, Brazil: reference values 10–65pg/mL).
All removed parathyroid tissue underwent complete histopathology analysis.
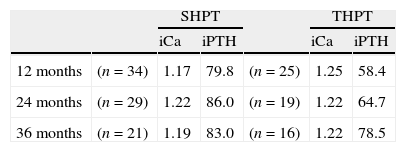
ResultsPre-operative data and laboratory findings are depicted in Table 1.
Pre-operative data and laboratory findings.
| G1 (n=66) | G2 (n=52) | |||
| SHPT | THPT | SHPT | THPT | |
| n | 38 | 28 | 29 | 23 |
| Age | 40.0 (14–58) | 44.0 (24–62) | 50.7 (26–74) | 44.4 (28–63) |
| Female/male | 24/14 | 14/14 | 11/18 | 13/10 |
| Years on dialysis | 7.7 (0.6–16) | 6.5 (2–20) | 7.9 (3–25) | 6.4 (0.8–13) |
| Years of renal graft | – | 2.8 (0.5–7) | – | 3.3 (0.4–10) |
| Pre-op iCa | 1.36a (1.19–1.83) | 1.59 (1.42–1.77) | 1.41a (1.21–1.59) | 1.58 (1.41–1.90) |
| Pre-op iPTH | 1618.7 (298–2500) | 442.2 (109–1758) | 1810.2 (561–3500) | 465.8 (181–2237) |
Reference values: iCa=1.20–1.40mmol/L; iPTH=10–65pg/mL.
Data expressed as mean and range.
iCa, serum ionized calcium; iPTH, intact parathyroid hormone.
Mean post-operative iPTH and iCa serum measurements during the follow-up of cured patients in both groups are shown in Tables 2 and 3.
Post-operative mean iCa and iPTH in cured patients in G1-group (conventional).
| SHPT | THPT | |||||
| iCa | iPTH | iCa | iPTH | |||
| 12 months | (n=34) | 1.17 | 79.8 | (n=25) | 1.25 | 58.4 |
| 24 months | (n=29) | 1.22 | 86.0 | (n=19) | 1.22 | 64.7 |
| 36 months | (n=21) | 1.19 | 83.0 | (n=16) | 1.22 | 78.5 |
Reference values: iCa=1.20–1.40mmol/L; iPTH=10–65pg/mL.
Data expressed as means.
SHPT, secondary hyperparathyroidism; THPT, tertiary hyperparathyroidism; iCa, serum ionized calcium; iPTH, intact parathyroid hormone.
Post-operative mean iCa and iPTH in cured patients in G2-group (stereomicroscopy).
| SHPT | THPT | |||||
| iCa | iPTH | iCa | iPTH | |||
| 12 months | (n=26) | 1.15 | 61.3 | (n=21) | 1.20 | 60.8 |
| 24 months | (n=18) | 1.09 | 113.2 | (n=14) | 1.18 | 65.0 |
| 36 months | (n=13) | 1.13 | 125.4 | (n=9) | 1.22 | 80.1 |
Reference values: iCa=1.20–1.40mmol/L; iPTH=10–65pg/mL.
Data expressed as mean.
SHPT, secondary hyperparathyroidism; THPT, tertiary hyperparathyroidism; iCa, serum ionized calcium; iPTH, intact parathyroid hormone.
Persistent hyperparathyroidism due to supernumerary parathyroid glands not recognized during surgical procedure was observed in two patients, one from each group; those patients were excluded from the study.
Definitive hypoparathyroidism was observed in 7/118 (5.9%) patients, 4/66 (6%) in G1, and 3/52 (5.7%) in G2.
Graft-dependent recurrence was observed in 4/118 (3.3%) patients, two in each group (G1, 2/66; 3.0% and G2, 2/52; 3.8%) (Table 4). All graft-dependent recurrences were observed in dialysis patients. None occurred in kidney-grafted patients.
Graft-dependent recurrence in patients from G1 (2/66) – conventional technique, and from G2 (2/52) – stereomicroscopy.
| 12 months | 24 months | 36 months | ||||
| iCa | iPTH | iCa | iPTH | iCa | iPTH | |
| Patient 1 (G1) | 1.46 | 13 | – | – | 1.29 | 1165 |
| Patient 2 (G1) | 0.90 | 22 | 1.29 | 104 | 1.56 | 168 |
| Patient 3 (G2) | 1.49 | 70 | 1.40 | 717 | 1.49 | 940 |
| Patient 4 (G2) | 1.21 | 224 | 0.97 | 1259 | – | – |
Reference values: iCa=1.20–1.40mmoL/L; iPTH=10–65pg/mL.
iCa, serum ionized calcium; iPTH, intact parathyroid hormone.
Surgery for renal hyperparathyroidism is performed in Brazil in relatively few medical centers, sometimes leading to long waiting times and, consequently, to worse clinical conditions at the time of surgery. Throughout the years, the authors have observed a worsening in medical conditions among renal patients at the time of surgery, when comparing patients from G1 (surgeries performed from 04/2000 to 05/2005) to G2 (surgeries performed from 03/2008 to 10/2009). As observed in SHPT patients from G2, serum calcium levels were higher than those seen in SHPT patients from G1 (t-test; p<0.05). This finding is in agreement with patients with a more severe disease and, moreover, these are indicators that patients in G2 underwent surgery in a worse condition. Thus, the apparently similar surgical results in graft-dependent recurrence observed between G1 and G2 (two recurrences in each group) could be assessed as a positive outcome for stereomicroscopy. The more severe disease observed in G2 may be the explanation for the finding of a similar recurrence in both groups. Since the macroscopic examination protocol used in G1 was performed by the same surgeon that also supervised the stereomicroscopy in G2, a fair comparison can be performed.
From the 118 overall patients, graft-dependent recurrence was observed only in four (3.3%). This can be considered a low graft-dependent recurrence rate compared to literature data.18–20 Considering the gravity of the illness observed in these patients, the experience of the surgeon probably contributed to such a positive outcome.
The clearly abnormal macroscopic findings observed in removed parathyroid indicate the severity of the illness and impose an especially difficult challenge in tissue selection. The effort for finding eligible areas for autotransplantation among abnormal parathyroid glands makes stereomicroscopy an interesting tool for its standardization. The search for stromal fat cells as previously described by Neyer et al.12 may provide a reproducible technique for tissue selection in attempts to reach low graft-dependent recurrences rates, even for surgeons not so experienced in parathyroid surgeries.
Another important observation is that all graft-dependent recurrences were in dialysis patients. None occurred in kidney-grafted patients. It is probable that the continuous exposure of the grafted parathyroid tissue to uremic environment may be the main driver for tumor recurrence in patients under long-term dialysis treatment, independent of the surgical technique employed.
Post-operative PTH measurements during the follow-up of cured patients were similar in G1 and G2, as were definitive hypoparathyroidism rates.
As mentioned above, persistent hyperparathyroidism due to supernumerary parathyroid glands not recognized during surgical procedure was observed in two patients, one in each group and those patients were excluded from the study. Occurrence of supernumerary and/or ectopic parathyroid glands is more frequent among chronic kidney failure patients,21 and this presents an important and additional challenge in surgical treatment of renal hyperparathyroidism.
Limitations are related to the heterogeneous characteristics of renal patients enrolled in this study, with their particular clinical and laboratory findings, and considering them as a group presents a real challenge, especially when a historical control is used. Another limitation is related to the severity of these patients: the marked illness and long-lasting renal condition makes them a special group, whose outcome would not be expected in ordinary patients with chronic renal disease. In addition, levels of 25-OH vitamin D were not available in this study.
ConclusionStereomicroscopy in renal hyperparathyroidism surgical treatment may provide a standardized parathyroid tissue selection for autotransplantation, and is potentially helpful in obtaining a low incidence of graft-dependent recurrence. Additional controlled and randomized studies are necessary to confirm these results.
FundingThis study was supported by FAPESP: N° 07/51056-5.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Ohe MN, Santos RO, Neves MCd, Carvalho AB, Kunii IS, Abrahão M, et al. Autotransplant tissue selection criteria with or without stereomicroscopy in parathyroidectomy for treatment of renal hyperparathyroidism. Braz J Otorhinolaryngol. 2014;80:318–24.