Minor structural alterations of the vocal fold cover are frequent causes of voice abnormalities. They may be difficult to diagnose, and are expressed in different manners. Cases of intracordal cysts, sulcus vocalis, mucosal bridge, and laryngeal micro-diaphragm form the group of minor structural alterations of the vocal fold cover investigated in the present study. The etiopathogenesis and epidemiology of these alterations are poorly known.
ObjectiveTo evaluate the existence and anatomical characterization of minor structural alterations in the vocal folds of newborns.
Methods56 larynxes excised from neonates of both genders were studied. They were examined fresh, or defrosted after conservation via freezing, under a microscope at magnifications of 25× and 40×. The vocal folds were inspected and palpated by two examiners, with the aim of finding minor structural alterations similar to those described classically, and other undetermined minor structural alterations. Larynges presenting abnormalities were submitted to histological examination.
ResultsSix cases of abnormalities were found in different larynges: one (1.79%) compatible with a sulcus vocalis and five (8.93%) compatible with a laryngeal micro-diaphragm. No cases of cysts or mucosal bridges were found. The observed abnormalities had characteristics similar to those described in other age groups.
ConclusionAbnormalities similar to sulcus vocalis or micro-diaphragm may be present at birth.
As alterações estruturais mínimas (AEM) da cobertura das pregas vocais são causas frequentes de alterações vocais. Podem ser de diagnóstico difícil, e expressam-se de modo variável. O cisto intracordal, o sulco vocal, a ponte de mucosa e o microdiafragama laríngeo constituem o grupo das AEM da cobertura das pregas vocais pesquisadas neste estudo. Sua etiopatogenia e epidemiologia não são bem conhecidas.
ObjetivoAvaliar a existência e a caracterização anatômica das AEM em prega vocal de neonatos.
MétodosForam estudadas 56 laringes excisadas de neonatos, de ambos os sexos. As laringes foram examinadas a fresco ou descongeladas após conservação por congelação, sob microscopia com aumento de 25 e 40×. As pregas vocais foram inspecionadas e palpadas por dois examinadores, com o intuito de encontrar AEM semelhantes às classicamente descritas e outras indeterminadas. As laringes com alterações foram submetidas a exame histológico.
ResultadosForam encontradas seis alterações em laringes distintas: uma (1,79%) compatível com sulco vocal e cinco (8,93%) compatíveis com microdiafragma laríngeo. Não foram encontrados cistos e pontes de mucosa. As alterações presentes apresentavam características semelhantes às descritas em outras faixas etárias.
ConclusãoAlterações semelhantes ao sulco vocal e ao microdiafragma laríngeo podem estar presentes ao nascimento.
In the contemporary world, the human voice is increasingly used in professional relationships, in the media, and in specific professions such as telemarketing operators, salesclerks, teachers, singers, actors, and others. Voice alterations can result from vocal behavior, through incorrect or improper use of the voice. Organic factors, such as small anatomical alterations of the larynx, may also predispose to dysphonia and vocal fatigue.
These small anatomical alterations of the larynx were first mentioned in the literature by Arnold in 1958, who described them as “minor anatomical changes” of the larynx.1 While studying patients with dysphonia, he classified small changes of the vocal folds, such as sulcus vocalis and asymmetries in a group, known as minor changes in the vocal folds.1 In 1994, Pontes and Behlau regrouped and classified these changes into four categories: laryngeal asymmetries, incomplete posterior fusion, deviations in glottal proportions, and changes in the vocal fold cover, under the name of minor structural alterations (MSAs).2,3
MSAs of the vocal folds are defined as small changes in configuration and structure of the larynx, which includes everything from simple anatomical variations to minor defects that do not have an impact on vital laryngeal functions, but that may or may not have an impact on the voice, according to their nature, location, extent, and vocal demands of the individual.3–6 These changes affect both men and women, adults and children, and include sulcus vocalis, epidermoid cysts, mucosal bridges, laryngeal micro-diaphragms, and vascular alterations.6–9
There are no epidemiological studies on their prevalence in the population and, since they constitute lesions that are difficult to diagnose clinically and are expressed at varying intensities, it is estimated that they are often underdiagnosed, but constitute one of the main causes of dysphonia in the adult population.10–12
Even considering all current studies and technological advances, many questions remain regarding MSAs, including those related to their etiology.13,14
Two theories, the congenital and the acquired, have been described to explain its etiopathogenesis. The first hypothesis considers that these lesions are congenital.15 During laryngeal development, congenital anomalies arising from changes in the fourth and sixth branchial arches give rise to the alterations in vocal fold cover.16 According to Bouchayer et al. cysts, sulcus vocalis, and mucosal bridges are often observed associated with one another, and might represent different stages of the same congenital entity.17,18
The second hypothesis is that these alterations are acquired secondary to a chronic inflammatory process in response to micro-trauma and diseases affecting the vocal folds.19 However, neither of the two theories completely explains the origin of MSAs. There are no studies in the literature on MSAs in newborns, and the finding of alterations in this age range could test the hypothesis that they may be congenital. Therefore, the aim of this study was to verify the presence of MSAs in neonatal larynges.
MethodsThe excised neonate larynges, obtained from the Faculdade de Ciências Médicas of Universidade Estadual de Campinas and sent for autopsy at the Department of Otorhinolaryngology, were studied in the dissection laboratory of the Discipline of Otorhinolaryngology.
This study included fresh larynges from autopsies of newborns up to seven days of life, of both genders, and all the specimens were preserved. Larynges that had a pinkish color, with intact, bright, and homogeneous mucosa were considered well preserved. Macerated larynges and those with lacerations and severe alterations in normal mucosa color were classified as inadequate.
Larynges of neonates whose time between death and autopsy was greater than 24h, those with prior tracheal intubation with macroscopically identifiable malformations, those preserved in formaldehyde, and those in poor preservation condition were excluded from the sample.
A total of 70 larynges were evaluated; seven were excluded due to preservation in formalin, five due to lacerations in the vocal folds caused by inadequate removal during necropsy, one due to prior intubation, and one showed macroscopically identifiable laryngeal malformation. Thus, 56 larynges were included in this study. Of the 56 larynges assessed, 23 were from a male child and 33 were from a female child. All were excised from stillbirths. The estimated gestational age ranged from 20 to 34 weeks, with a mean of 27.2 weeks. The time between death and autopsy ranged from two to 24h.
The larynges were assessed fresh, or were frozen immediately after autopsy and kept under refrigeration at −8°C for analysis within a 30-day period. The frozen larynges were thawed at room temperature one hour before the analysis. Of the 56 larynges assessed, nine were fresh and 47 were analyzed after freezing and thawing. The freezing time varied from one to 30 days.
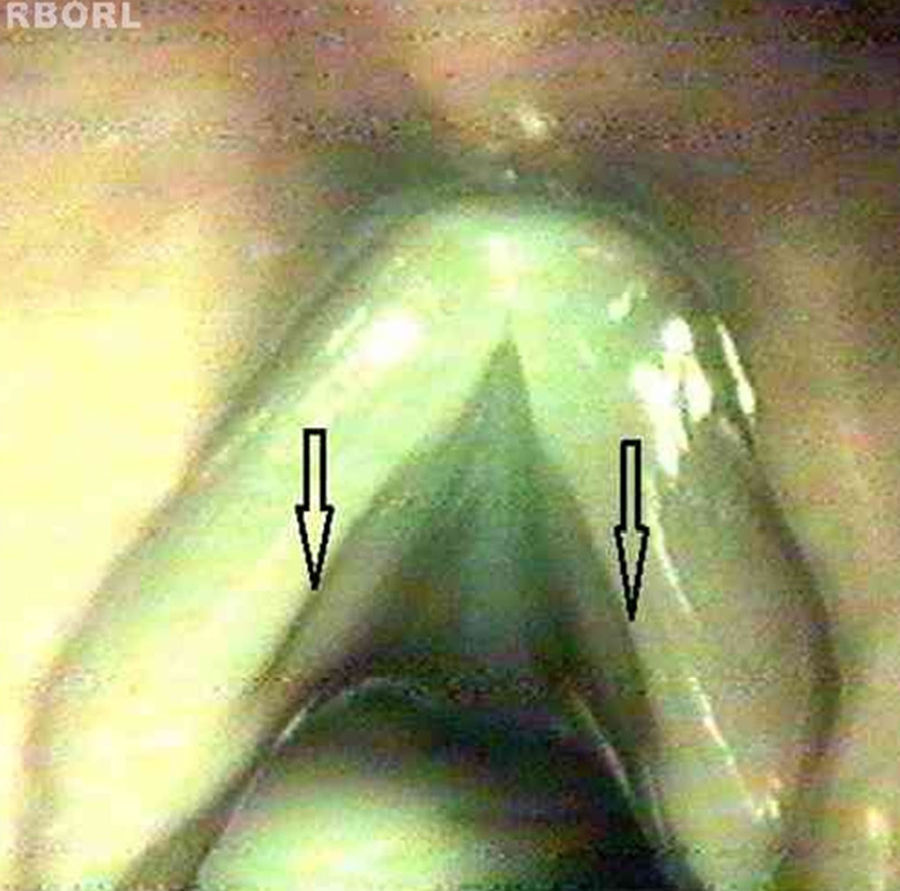
For the physical examination of the vocal folds under the microscope, the sulcus vocalis was considered as a longitudinal fusiform depression parallel to the free border of the vocal folds, of varying extension and depth; the cyst, as a circumscribed submucosal nodular structure of variable size with similar appearance to the classically described cyst; the mucosal bridge, as a mucosal handle inserted along the vocal folds, and the laryngeal micro-diaphragm, as a small membrane between the two vocal folds, located in the commissure and not exceeding one third of their length.
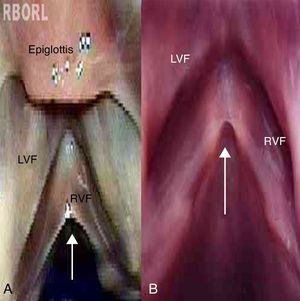
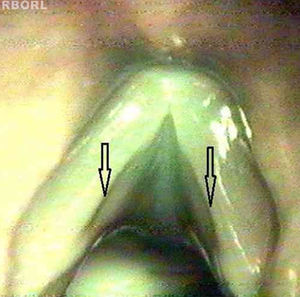
Cohen classifies as type I membranes affecting less than 35% of the glottal length.20 There is no precise delimitation between a micro-diaphragm and membranes that can be classified as Cohen type I. Therefore, this study grouped micro-diaphragms with characteristic appearance (Fig. 1b) and larger diaphragms extending up to 1/3 of the glottal length (Cohen type I – Fig. 1a).
The examination of the larynges involved two stages: inspection and instrumental palpation.
Inspection- 1
Larynges were arranged on the dissecting bench and inspected under light microscopy with 6× magnification, assessing the upper portion of the vocal folds, the anterior commissure, the posterior commissure, the ventricles, and the supraglottic region;
- 2
Larynges were then opened in the midline, posteriorly, to expose the vocal folds, aiming to preserve the soft tissues;
- 3
A methodical inspection of the upper portion, free border, and lower portion of the vocal folds was performed, as well as of the anterior commissure, under optical microscopy with magnification of 25× and 40×, which was also repeated using a green light filter to highlight vessels.
The vocal folds were palpated using a surgical blade with a blunt tip and a surgical dissector. Palpation was performed by carefully sliding the instrument along the upper portion, the free border, the lower portion of the vocal folds, and the anterior commissure, aiming to detect changes in the homogeneity, consistency of shape, and surface uniformity. Inspection and palpation were repeated after instillation of one drop of methylene blue solution, used to highlight possible surface irregularities, through the deposition of the colored liquid.
Larynges were always evaluated by two examiners simultaneously, comparing their observations and accepting their common opinion.
Upon inspection and instrumental palpation, the larynges were examined regarding:
- a)
the presence of MSAs;
- b)
the characterization of alterations found, compared to those classically described: sulcus vocalis, cysts, mucosal bridges, and laryngeal micro-diaphragms;
- c)
the characterization of other alterations eventually found, other than those classically described;
- d)
the location of alterations in the membranous portion of the vocal fold when divided into three equal parts of the vocal process at the anterior commissure, as anterior, middle, and posterior thirds (Fig. 2);
- e)
number of alterations;
- f)
association between two or more alterations.
The examination was filmed and recorded on VHS tape for reassessment of the material, if necessary, and for comparative purposes. The results were recorded in forms that were specifically prepared for this study, containing gender, newborn weight, gestational age, time between death and autopsy, cause of death, condition of the larynx, preservation method, and schematic representation of membranous portion of the vocal folds and evaluated segments. The images of the larynges that showed alterations were digitized and edited on a computer, using the Corel Photo-Paint program, release 10, applying the effect to highlight the relief.
Larynges that showed minor anatomical alterations were selected for histological study. They were fixed in 10% formalin for one to eight weeks and embedded in paraffin. The serial cross-sections were carried out in the longitudinal axis of the vocal folds, with 6-μm thickness and at 1-mm intervals from the posterior to the anterior commissure. The samples were stained with hematoxylin-eosin. The slides obtained were studied together with the pathologist.
The histological analysis of the sulcus vocalis used the criteria defined by Nakayama et al. (1994).21 The sulcus vocalis was considered as an isolated invagination of the squamous epithelium in the vocal fold mucosa, with greater depth than the total epithelium thickness. Irregularities near the areas of transition of squamous epithelium with respiratory epithelium were disregarded. Only invaginations deeper than the epithelium thickness were considered, in order to prevent incorrect interpretations resulting from artifacts caused by slide preparation.
Vocal cyst was histologically considered as a closed cavity, located in the lamina propria of the vocal fold mucosa, lined by stratified squamous epithelium and containing keratinized material. Mucosal bridge was histologically defined as a band of connective tissue lined by stratified epithelium. No histological criteria in the literature for laryngeal micro-diaphragm were found. Thus, the anatomical aspect of this alteration was used as a diagnostic criterion. As this was a descriptive and qualitative study, statistical assessments were performed as percentages. This study was approved by the Research Ethics Committee of Faculdade de Ciências Médicas of UNICAMP.
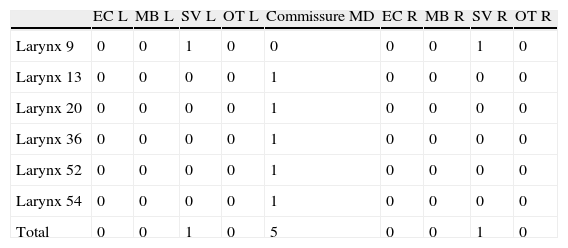
ResultsAlterations were found in six larynges (Table 1).
Numerical distribution of minor structural alterations found in six larynxes, regarding type and location, left or right vocal fold, and commissure (n=56).
| EC L | MB L | SV L | OT L | Commissure MD | EC R | MB R | SV R | OT R | |
| Larynx 9 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Larynx 13 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Larynx 20 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Larynx 36 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Larynx 52 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Larynx 54 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Total | 0 | 0 | 1 | 0 | 5 | 0 | 0 | 1 | 0 |
CE, epidermoid cyst; MB, mucosal bridge; SV, sulcus vocalis; OT, others; MD, Micro-diaphragm; L, left; R, right.
The alterations were:
- a)
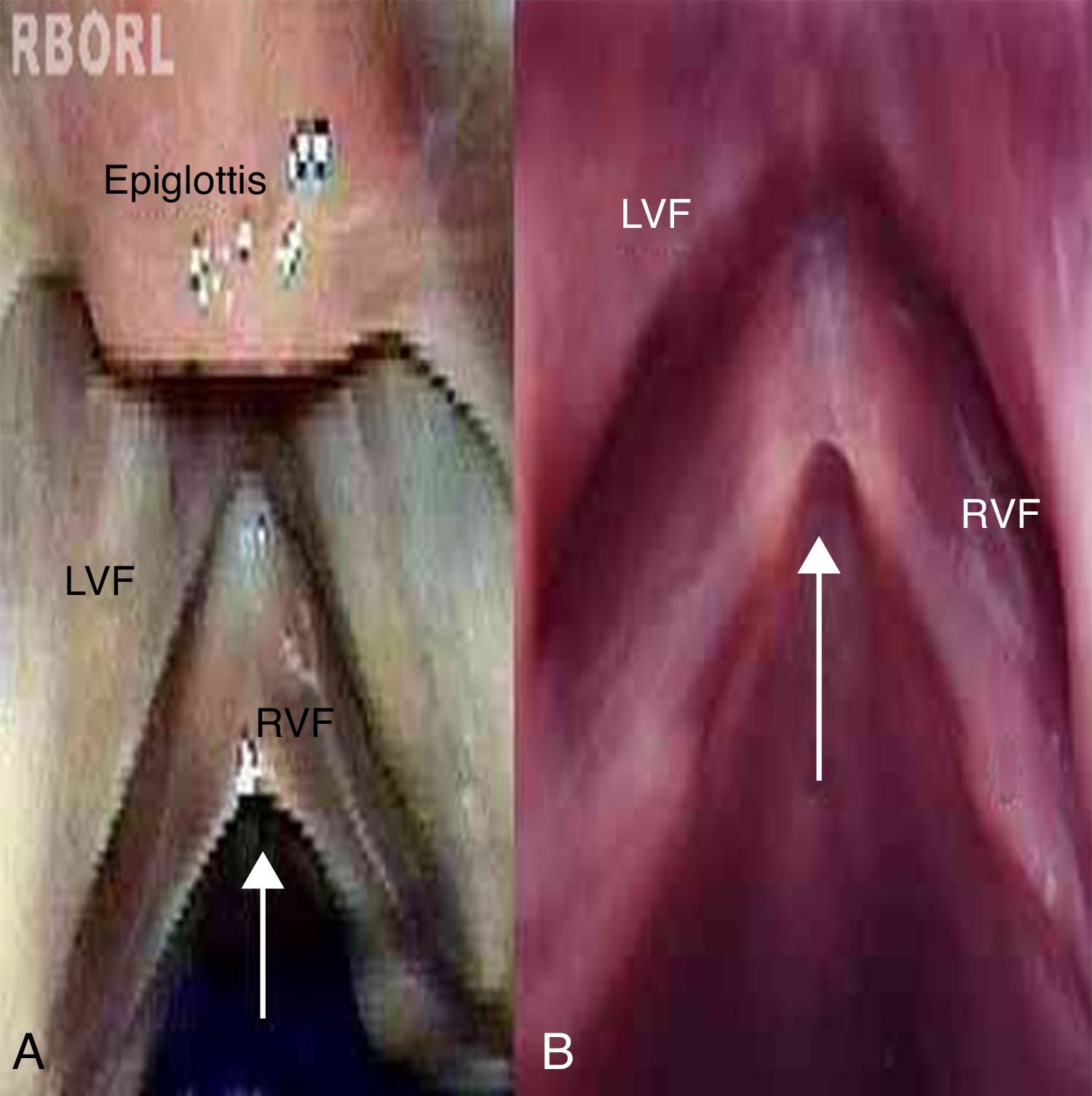
Larynx 9: longitudinal depression along the free border of both vocal folds, observed under the microscope as a broad, fusiform, darkened band, similar to type I sulcus vocalis. The instrumental palpation showed relief alteration when the longitudinal depression was palpated (Fig. 2).
The distribution of the alterations found according to their location is shown in Table 2. The distribution of MSAs found according to gender is shown in Table 3.
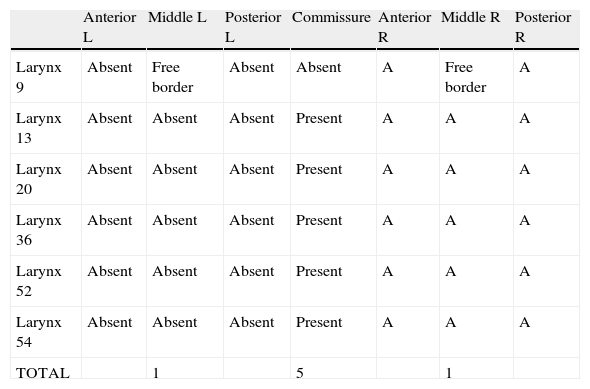
Distribution of the six alterations found according to their location in the anterior, middle, and posterior thirds of the membranous portion of the vocal folds, left and right, anterior commissure, upper and lower portion, and free border of the vocal folds (n=56).
| Anterior L | Middle L | Posterior L | Commissure | Anterior R | Middle R | Posterior R | |
| Larynx 9 | Absent | Free border | Absent | Absent | A | Free border | A |
| Larynx 13 | Absent | Absent | Absent | Present | A | A | A |
| Larynx 20 | Absent | Absent | Absent | Present | A | A | A |
| Larynx 36 | Absent | Absent | Absent | Present | A | A | A |
| Larynx 52 | Absent | Absent | Absent | Present | A | A | A |
| Larynx 54 | Absent | Absent | Absent | Present | A | A | A |
| TOTAL | 1 | 5 | 1 |
L, left; R, right.
The methylene blue solution was embedded, in most cases, on the laryngeal ventricles and anterior commissure, enhancing their anatomy. In larynx 9, the solution highlighted the sulcus vocalis, which had been previously visualized. In the other analyzed larynges, the solution did not help in the identification of surface alterations that had not been previously visualized.
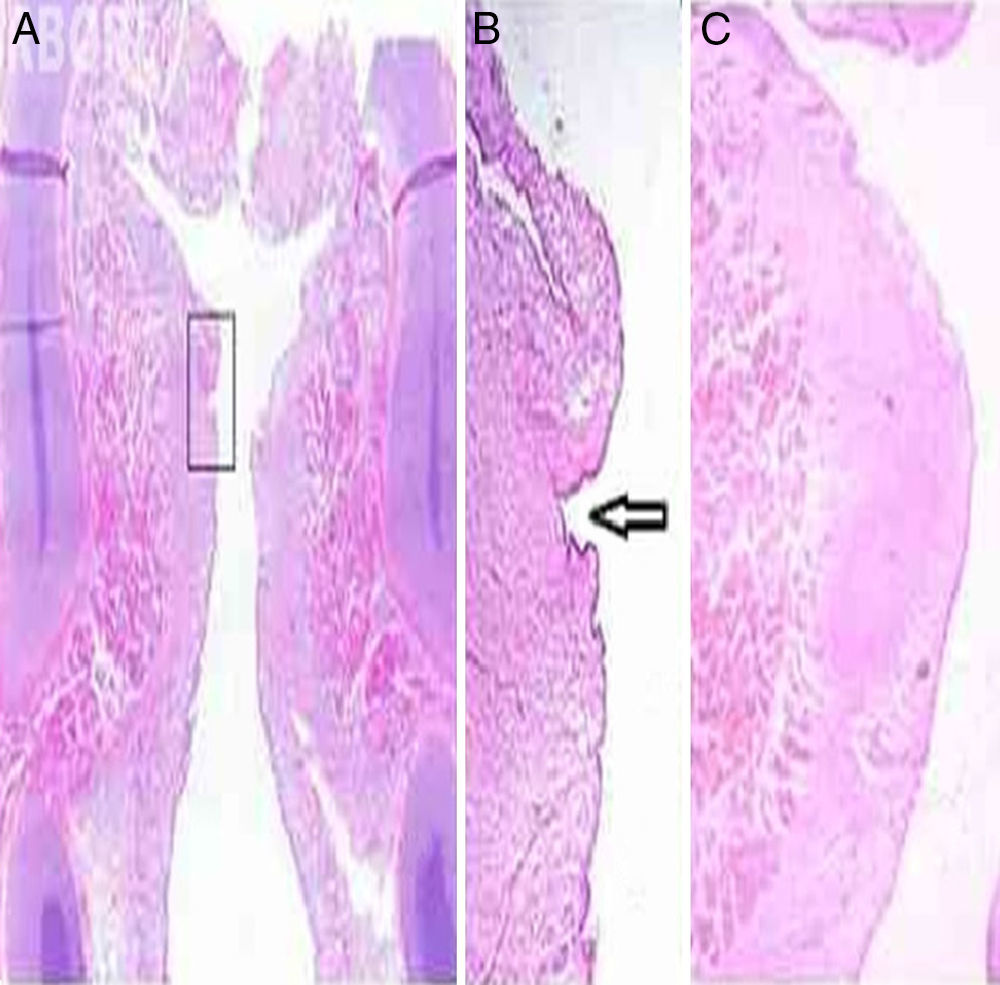
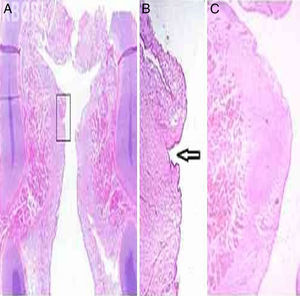
Histological examination corroborated the examination by inspection and palpation of larynx 9, whose histological analysis was compatible with type I sulcus vocalis in the left vocal fold, according to the criteria suggested by Nakayama et al. (1994)21 (Fig. 3 A and B). The sulcus vocalis observed in the right vocal fold could not be histologically demonstrated. Fig. 3C shows the histological aspect of the vocal fold in newborns. No alteration associations were found.
(A) Frontal histological section of larynx 9, HE staining. In the detail, (B) left sulcus vocalis, indicated by the arrow (up to 20×). (C) Frontal histological section of left vocal fold without alterations, in larynx 2, which showed no alteration at the inspection and instrumental palpation examination, HE staining, 12× magnification.
The alteration similar to type I sulcus vocalis found in larynx 9 was characterized histologically by an invagination of the stratified epithelium in the lamina propria, in the region of contact between the vocal folds, far from the region of transition between the stratified and respiratory epithelium, and could be observed only in the left vocal fold in several sections performed in the middle third of the vocal folds. Sections performed in the anterior and posterior thirds showed invaginations in the same region, albeit superficial, which did not meet the criteria of Nakayama et al. (1994) for sulcus vocalis.21
Of the five diaphragms, four had the classic aspect of micro-diaphragm and one had the classic aspect of Cohen type I diaphragm.
The histological study of the larynges with laryngeal micro-diaphragms was not satisfactory, due to the technical difficulties in obtaining perfect parallelism in longitudinal sections, necessary to demonstrate the micro-diaphragms. No cysts and mucosal bridges were found in the larynges studied.
DiscussionLiterature is still limited in relation to the presence of sulcus vocalis, mucosal bridge, cyst, and laryngeal micro-diaphragm, and although there are some studies dating from the late 19th century and early 20th century, most of the publications date from the 1980s to the present day.5 This is due to the development of diagnostic techniques, with the emergence of flexible endoscopy, and the development and dissemination of stroboscopy.12 Even with the technological advances and the intensification of studies, many questions remain to be answered about MSAs of the larynx.
There are two theories that attempt to explain the etiopathogenesis of some MSAs: congenital and acquired. The congenital theory presupposes that MSAs may be due to small defects occurring during larynx embryogenesis, resulting from changes in the fourth and sixth branchial arches. This theory is based on the early onset of dysphonia in some patients with MSAs;5,18 the presence of associated alterations, such as cysts, sulci, and mucosal bridges;5,6,18 the observation that the lesions did not reappear after their surgical excision;5,18 the presence of alterations in several individuals of the same family,18 and the presence of sulcus vocalis in other animals such as giraffes, deer, antelope and pigs, described by several authors,1 with regressive significance of these lesions in humans.
According to Bouchayer et al. (1985), the release of the keratinized contents of the epidermoid cyst on the free border of the vocal fold results in a groove, while its opening at the superior and inferior portions of the vocal fold results in the formation of the mucosal bridge. This hypothesis can explain the genesis of unilateral lesions, but it appears to be unlikely in bilateral lesions of sulcus vocalis or mucosal bridge, as it would require the bilateral rupture of the cysts, symmetrically.18
The acquired theory presupposes that the MSAs originate in response to external stimuli. This hypothesis is based on the following observations: lesions such as epidermoid cysts are mostly found in the transition area between the anterior and the posterior third of the vocal fold, considered the segment of greater functional activity of the vocal fold7; the presence of inflammatory reaction around the lesions, such as sulcus vocalis and epidermoid cysts observed in the histological assessment18; the frequent association of abuse and/or misuse of the voice in patients with these lesions; the late onset of dysphonia observed in some patients8; and the presence of alterations such as sulcus vocalis found in excised larynges during laryngectomy in patients with malignant neoplasm of the larynx, suggesting that the chronic inflammatory process related to neoplasms can lead to sulcus vocalis formation.18
As shown in the literature, neither of the two theories of the etiopathogenesis of MSAs of the vocal folds can explain the origin of each individual lesion. There are no conclusive epidemiological studies on MSAs in the population. This is due to the great variability of these alterations and their presentation, as they are often nearly asymptomatic. Moreover, their diagnosis is also difficult. The mucus that covers the vocal folds flattens the surface irregularities and makes it difficult to identify alterations, requiring evaluation under microscopy and palpation of the vocal folds to identify the alteration.
Monday et al. stated that intracordal cysts are not unusual, although they are not frequently mentioned in the literature; but with the evolution of diagnostic methods, these lesions have become more easily identified.5 Arnold, in 1958, found 12 individuals with sulcus vocalis in a group of 1250 adult soldiers with dysphonia. He comments that it is much easier to find individuals with laryngeal alterations in a group of dysphonic patients, and concludes that statistical studies can be greatly influenced by selective factors.1
Milutinovic and Vasiljevic (2001) found only 11 cases of sulcus vocalis in 1550 patients submitted to surgery, which represents only 0.7%. Therefore, it is possible that a large number of MSAs are not diagnosed during routine endoscopic assessment.9
There are no studies in the literature on MSAs in neonates. The only reported case of cystic lesion was described by Smith et al. in 2000, while reporting the presence of a cyst, described as having mucosal content and therefore not compatible with an epidermoid cyst in a neonate.5 The embryological treatises are vague in describing the mechanisms of vocal fold formation,13 and most authors, although they mention that the sulcus vocalis, epidermoid cysts, and mucosal bridge can be generated by defects of the fourth and sixth branchial arches, do not provide the theoretical basis to support this hypothesis.5,8,18
The formation of the larynx is completed around the tenth gestational week; as the present sample consisted of neonates older than 20 weeks of gestation, stillborn, this fact practically eliminates the influence of external factors, demonstrating that these results are important from the etiopathogenic point of view, suggesting a congenital etiology.
In this study, the finding of sulcus vocalis in larynx 9, excised from a neonate, reinforces the hypothesis that this alteration is of congenital origin. This is the first description of a sulcus vocalis in a neonate. In this case, the possibility that it was a pseudo-sulcus vocalis, which consists of a grooved image in the mucosa throughout the entire vocal fold, resulting in infra-glottic edema, was considered; however, evidence of the lesion and histological findings ruled out this possibility.
Bouchayer et al. (1985) already supported the congenital theory to explain the origin of the sulcus vocalis. They suggest that they originate from intracordal cysts, through the exteriorization of their content.18 However, the present results suggest that these lesions may already be present at birth, constituting an independent entity, regardless of the epidermoid cyst.
One difficulty found in this study was the lack of histological standardization for MSAs in neonate vocal folds, as the studies on the histological aspects of the MSAs are rare and refer to adults.20 Hirano and Sato (1993),10 in their histological atlas of the human larynx, report that the layer structure in neonates differs significantly from that of adults. The biggest difference is observed in the lamina propria of the mucosa. In neonates, the entire lamina propria is very rudimentary and has a disarrayed aspect. It resembles the superficial layer of the vocal fold lamina propria of the adult, and consists in amorphous substance, scattered fibroblasts, collagen and elastic fibers. There are fewer collagen fibers, and more fibroblasts in the lamina propria of neonates than in the superficial lamina propria of adults. From this mechanical point of view, the entire mucosa can be considered as and the vocal muscle as body.
Therefore, the histological classification of sulcus vocalis in neonates, as well as in adults, is not possible. Because it is an invagination that lacks adherence to the vocal muscle, the abnormality in larynx 9 was classified as suggestive of type I sulcus only in the left vocal fold. The authors did not observe histological changes in the right vocal fold. This is probably due to the difference in depth of the invagination of the epithelium in the lamina propria. Thus, the importance of relating the macroscopic findings with the histological analysis is also emphasized.
The nine larynges analyzed as fresh specimens were frozen and thawed for a second assessment. Both evaluations were filmed and recorded for comparison. No differences were observed between the two assessments, and characteristics of texture, color, sheen, and elasticity, as well as the presence of mucus covering the entire larynx, remained unchanged. The histological study also showed no alterations in the larynges that were frozen and thawed. Thus, freezing is a suitable option for newborn larynx conservation for anatomical studies. It was decided not to study vascular alterations. This assessment should be performed in vivo, as organ exsanguination in an excised larynx complicates the assessment of small blood vessels.
The studies on sulcus vocalis, based on autopsies, have very different patient populations. In 1967, Ishii et al. identified five cases of sulcus vocalis in 200 larynges excised at autopsy (2.5%).14 In 1976, Shin also found five cases of sulcus vocalis, but in 1200 excised larynges (0.42%). In 1994, Nakayama et al.21 reported 48% of histological alterations compatible with sulcus vocalis in excised larynges of patients with cancer of the larynx. In 2000, Ming et al. reported that among the 72 larynges examined histologically, 23% showed alterations compatible with sulcus vocalis.15 In the present study of 56 neonate larynges, sulcus vocalis was found in one (1.78%). Similar studies with larger samples are needed to complement this study.
The finding of MSAs in neonates reinforces the theory that they are congenital alterations. However, there are congenital lesions with late clinical expression, such as the thyroglossal cyst and branchial cyst. The fact that MSAs were not found in neonates, such as mucosal bridges and cysts, does not rule out the possibility that these alterations are congenital, as they may have a late clinical expression. It is also possible that the alterations show distinct characteristics at birth and undergo modifications over time due to external factors that cause tissue inflammation. The small sample size does not allow the congenital theory to be ruled out for other types of MSAs that were not found in this study.
There are few studies in the literature on laryngeal micro-diaphragms (Ford et al. 1994).8 However, this was the most frequent alteration in the present study, occurring in 8.93% of 56 larynges evaluated. As previously mentioned, it is likely that these changes, as well as other MSAs, often go undiagnosed. According to Wang (2000), the vocal folds are formed in adduction and are medially united by the epithelial lamina; alterations in the cavitation process and cell necrosis of the epithelial lamina, which is completed around the eighth week of the gestational process, can generate small defects such as laryngeal membranes.19 The finding of five laryngeal micro-diaphragms in the present study confirms the congenital origin of these alterations.
ConclusionsBased on the assessment of 56 excised larynges from neonates, it is concluded that there are alterations in the vocal folds that resemble MSAs described in adult larynges in some of their forms, and when they are studied by optical microscopy, they show the same characteristics as those in adults. Of the MSAs selected for the study, laryngeal micro-diaphragm and sulcus vocalis were found, and the first was the most frequent alteration.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Silva AR, Machado Júnior AJ, Crespo AN. Anatomical study of minor alterations in neonate vocal folds. Braz J Otorhinolaryngol. 2014;80:311–7.