Chronic Rhinosinusitis with Polyps (CRSwNP) is characterized by high heterogeneity and postoperative recurrence rate. This study aims to explore the clinical significance of tissue Leukocyte-Specific Transcript 1 (LST1) in predicting CRSwNP recurrence.
MethodsWe enrolled 62 CRSwNP patients including 30 primary CRSwNP and 32 recurrent CRSwNP patients, and 40 Healthy Controls (HC). Tissue samples were collected. Tissue LST1 expression was assessed by Reverse Transcription-Polymerase Chain Reaction (RT-PCR), Western Blotting (WB) and Immunofluorescence (IF) staining. The predictive values of LST1 expression for CRSwNP postoperative recurrence were assessed through the Receiver Operating Characteristic (ROC) curves.
ResultsThe tissue levels of LST1 were significantly increased in the CRSwNP group than the HC group, especially in the recurrent group, and the elevated LST1 mRNA levels were positively correlated with the peripheral eosinophil percentages, tissue eosinophil counts and percentages. IF staining results showed that the LST1 protein levels were higher in CRSwNP patients, especially in the recurrent patients than in the HC group. ROC curves highlighted that tissue LST1 levels were associated with recurrent CRSwNP and exhibited a higher predictive ability for postoperative CRSwNP recurrence.
ConclusionThis was the first report suggesting that LST1 expression was upregulated and associated with mucosal eosinophil infiltration and CRSwNP recurrence. Tissue LST1 could be a promising biomarker for predicting postoperative recurrence in CRwNP patients.
Level of evidenceLevel 5.
Chronic Rhinosinusitis with Nasal Polyp (CRSwNP) is a common chronic disease with inflammatory pathological changes in the nasal cavity and sinus mucosa. It has affected about 15% of the adult population worldwide.1,2 CRSwNP is generally concomitant with other disorders, such as asthma and allergic rhinitis,3,4 and significantly impacts the quality of life. Previous studies demonstrated that T-helper 2 (Th2) cell-driven eosinophilic inflammation was predominant in the underlying pathomechanism of CRSwNP and contributed to its postoperative recurrence.5–8 Although the surgery was of great benefit for symptom alleviation, up to 40% of patients with CRSwNP still experienced recurrence within 12-months after surgery.9 Given that, predicting postoperative recurrence is vital in guiding the options for personalized treatments and improving follow-up protocols. Hence, it is essential to find objective indicators or biomarkers which can be used to predict postoperative recurrence of CRSwNP.
Leukocyte-Specific Transcript 1 (LST1), the human homologue of the murine B144 gene, is a pleiotropic biomolecule encoded by the LST1 gene and express in multiple immune cells, especially in Dendritic Cells (DCs) and T-cells.10,11 Previous study demonstrated that LST1 expression appeared to be regulated during inflammation and elevated expression of LST1 mRNA isoforms was detected in cell lines after stimulation with pro-inflammatory compounds.12 A recent study demonstrated that LST1 was pivotal in DCs mediated antigen presentation, Th2 cell differentiation and eosinophil movement.12 Therefore, we assumed that LST1 might be involved in the pathological process of CRSwNP and contributed to predicting postoperative recurrence.
In this study, we aim to explore expression levels of LST1 in the tissue mucosa in the Health Control (HC) group and CRSwNP group, including primary CRSwNP group and recurrent CRSwNP group, to assess its potential value in predicting postoperative recurrence.
MethodsParticipants and settingsIn the present study, 44 HC subjects and 62 CRSwNP patients including 30 primary and 32 recurrent patients were recruited between July 2019 and December 2019 in our department. Of these, patients who underwent surgery for anatomic variations or cerebrospinal rhinorrhea and had no other sinus disease were recruited into the HC group. Patients with nasal polyps who underwent functional endoscopic sinus surgery were enrolled in the CRSwNP group. All patients met the diagnostic criteria of CRSwNP according to the guidelines of the European Position Paper on Rhinosinusitis and Nasal Polyps 2012.13 Exclusion criteria in this study were listed as following: (1) Age <18 or >65 years; (2) Patients with other nasal or sinus diseases, such as fungal sinusitis, allergic fungal rhinosinusitis, cystic fibrosis and malignancy; (3) Patients with a diagnosis of inflammatory diseases, autoimmune diseases; (4) Treated with immunotherapy, antibiotics, nasal or systemic corticosteroids, or anti-allergic drugs within 4-weeks before the surgery; (5) Severe heart and kidney dysfunction; (6) Aspirin intolerance and (7) Pregnant condition. The diagnosis of recurrent CRSwNP was made when patients met these two criteria: (1) Presence of nasal polyps detected by nasal endoscopy and/or Computed Tomography (CT) after surgery; (2) Symptoms lasting for more than 1 week despite treating with normative intranasal corticosteroid.14 All subjects underwent routine preoperative examination, blood tests, nasal endoscopy, chest X-Rays, electrocardiography and High-Resolution Computed Tomography (HRCT) or Magnetic Resonance Imaging (MRI). All CRSwNP patients scored their nasal symptoms using the widely accepted Visual Analogue Scale (VAS) as previously described.15 Lund–Kennedy scoring system was used for preoperative nasal endoscopy assessments.16 During surgery, polyp tissues from CRSwNP patients and middle turbinate mucosal tissues from HCs were collected.
qRT-PCR analysisTissue RNA was extracted with Trizol reagent (Thermo Fisher Scientific, Shanghai, China), and The RNA concentration was measured by Nanodrop 2000c (Thermo Fisher Scientific, Shanghai, China). RNA was reverse transcribed into cDNA using SureScript First-strand cDNA synthesis kit (US EVERBRIGHT, Suzhou, China), according to the manufacturer's instructions. qRT-PCR was performed using 100ng of cDNA with the SYBR Green qPCR Supermix (US EVERBRIGHT, Suzhou, China) to monitor DNA synthesis using specific primers. The primers used were shown as followed: LST1: Forward 5’-TCGCCTCCCATCAGCACCTTCTGT-3’, Reverse 5’-TGGGGTGCTCAGGTGGGTTTGT-3’; GAPDH: Forward 5’-CTCCTCCTGTTCGACAGTCAGC-3’, Reverse 5’-CCCAATACGACCAAATCCGTT-3’.
Western blotting (WB)Collected tissue specimens were completely lysed in RIPA buffer, then centrifuged and the supernatant was collected. The protein concentrations were detected by the BCA protein kit (New Cell & Molecular Biotech, Suzhou, China) according to the instructions. Separated the extracted protein by gel electrophoresis (Beyotime Biotech, Shanghai, China) and blotted onto PVDF membranes (0.2μm). The PVDF membrane was sealed with 5% non-fat milk for 1h, then incubated with the primary antibodies anti-LST1 (Proteintech, Wuhan, China) and anti-GAPDH (Affinity Biosciences, Changzhou, China) overnight at 4°C. Then, the membranes were incubated with a corresponding HRP-conjugated (1:3000) secondary antibody for 1h at room temperature. The blot bands were examined by Molecular Imager Chemidoc XRS System (UVP, Ltd, USA), and the band densities were quantified with a computerized densitometer.
IF staining of LST1For IF staining, the tissue sections were first stained with specific primary antibodies overnight at 4°C. After washing, the tissue sections were incubated with Alexa Fluor 568 – and/or Alexa Fluor 488 – conjugated secondary antibodies at room temperature for 2h. Details can be found in the reference.17
Statistical analysisContinuous variables with normal distribution were described as mean±Standard Deviation (SD). For normally distributed variables, Student's t-test was applied to compare the difference. Otherwise, the Kruskal-Wallis H test or Mann-Whitney U test was used. To evaluate the correlation between tissue LST1 mRNA level and clinical variables, the Spearman correlation test was conducted. Receiver Operating Characteristic (ROC) curves were performed to determine the predictive value of tissue LST1 mRNA levels and other indicators. All statistical analyses were conducted on the SPSS statistics software version 25.0 (IBM, Chicago, IL, USA), and figures were constructed in GraphPad Prism 8.0 (Software Inc. La Jolla, CA, USA). For all tests, p<0.05 was considered statistically significant.
ResultsDemographic and baseline characteristics of the study populationThe demographic and clinical data of all participants were displayed in Table 1. The rate of allergic rhinitis and asthma, blood eosinophil counts and percentages were significantly elevated in the CRSwNP group compared to the HC group (All p<0.05). However, other variables including gender, age, and BMI were no statistical difference between the two groups. As shown in Table 2, the patients with recurrent CRSwNP had a higher rate of allergic rhinitis, tissue eosinophil counts and percentages, and VAS score and Lund-Kennedy score than those primary CRSwNP patients (All p<0.05), but no statistical difference was observed between two groups in the other variables.
The demographic and clinical characteristics of subjects.
| Parameters | HC (n=44) | CRSwNP (n=62) | p-value |
|---|---|---|---|
| Age, years | 36.9±15.1 | 39.8±16.2 | 0.352 |
| Gender (male/female) | 29/15 | 20/42 | 0.843 |
| BMI, kg/m2 | 23.4±2.7 | 22.9±3.0 | 0.455 |
| Allergic rhinitis(yes/no), n | 0/44 | 40/22 | < 0.001 |
| Asthma(yes/no), n | 0/44 | 8/54 | 0.013 |
| Blood eosinophil counts (109/L) | 0.1±0.1 | 0.3±0.2 | 0.001 |
| Blood eosinophil percentages (%) | 2.4±1.3 | 4.9±4.0 | < 0.001 |
CRSwNP, Chronic Rhinosinusitis with Nasal Polyps; HC, Healthy Control; BMI, Body Mass Index.
The demographic and clinical characteristics of subjects.
| Parameters | Primary CRSwNP (n=30) | Recurrent CRSwNP (n=32) | p-value |
|---|---|---|---|
| Age, years | 38.4±17.5 | 41.1±15.0 | 0.511 |
| Gender (male/female) | 23/7 | 19/13 | 0.146 |
| BMI, kg/m2 | 22.7±3.0 | 23.2±2.9 | 0.495 |
| Allergic rhinitis(yes/no), n | 11/19 | 11/21 | 0.851 |
| Asthma(yes/no), n | 4/26 | 4/28 | 0.922 |
| Blood eosinophil counts (109/L) | 0.3±0.2 | 0.3±0.3 | 0.461 |
| Blood eosinophil percentages (%) | 4.6±3.2 | 4.8±4.4 | 0.826 |
| Tissue eosinophil counts, n/HPF | 19.1±13.0 | 28.3±21.2 | 0.042 |
| Tissue eosinophil percentages (%) | 10.4±8.7 | 16.8±13.0 | 0.027 |
| VAS score | 5.4±1.7 | 6.4±1.5 | 0.012 |
| Lund-Kennedy score | 7.2±2.7 | 9.6±2.9 | 0.001 |
CRSwNP, Chronic Rhinosinusitis with Nasal Polyps; BMI, Body Mass Index; VAS, Visual Analog Scale; HPF, High Power Field.
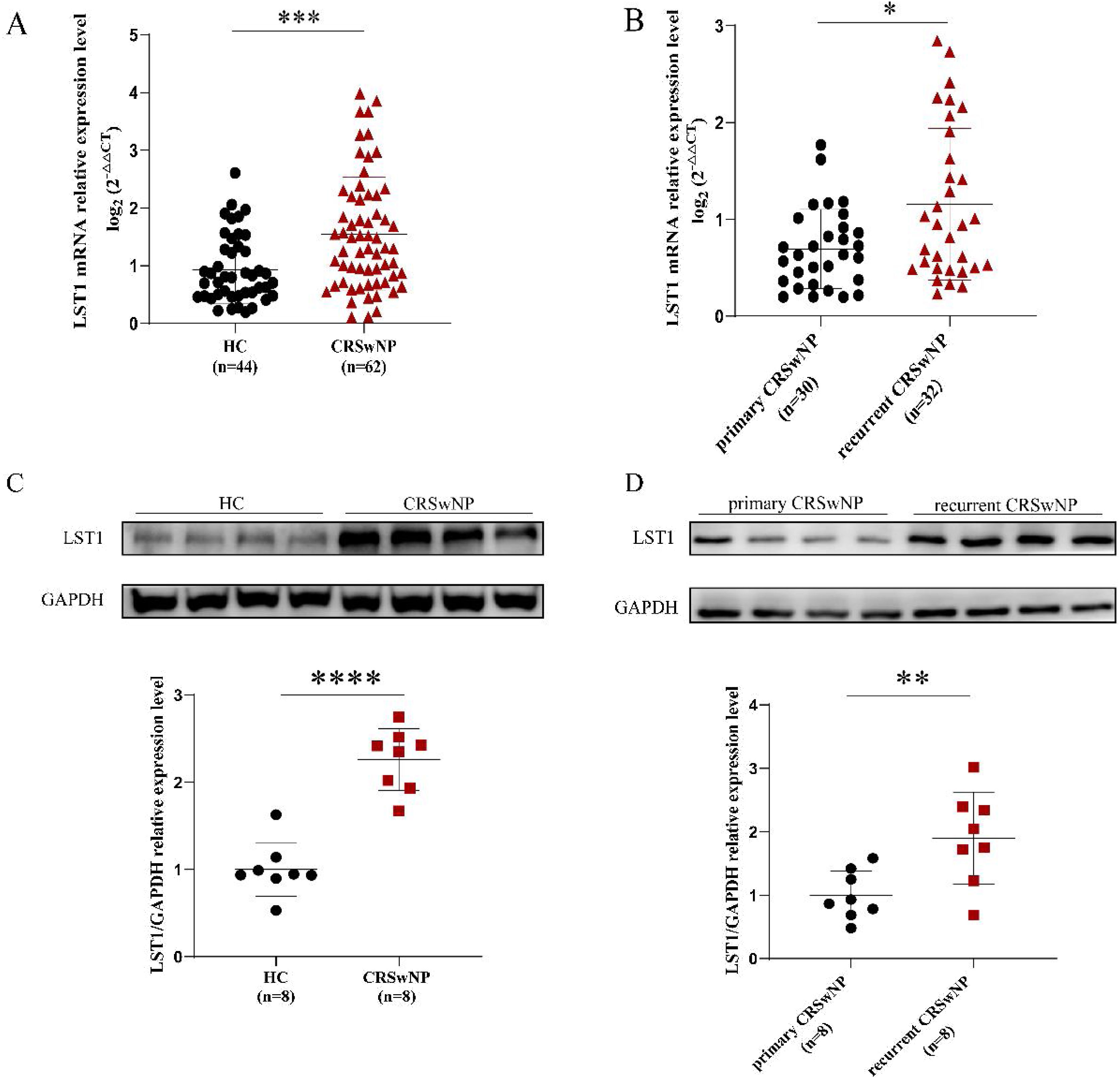
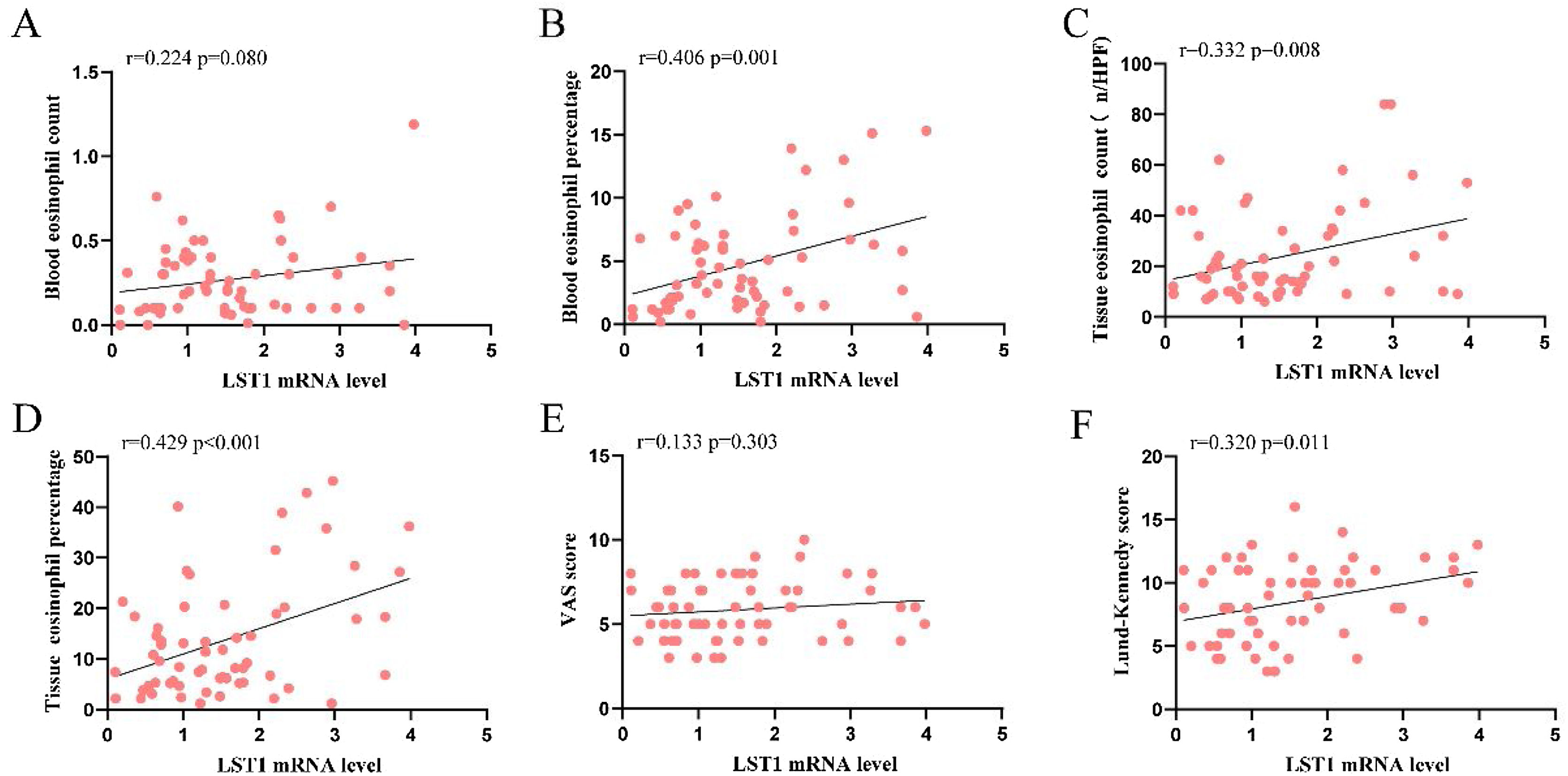
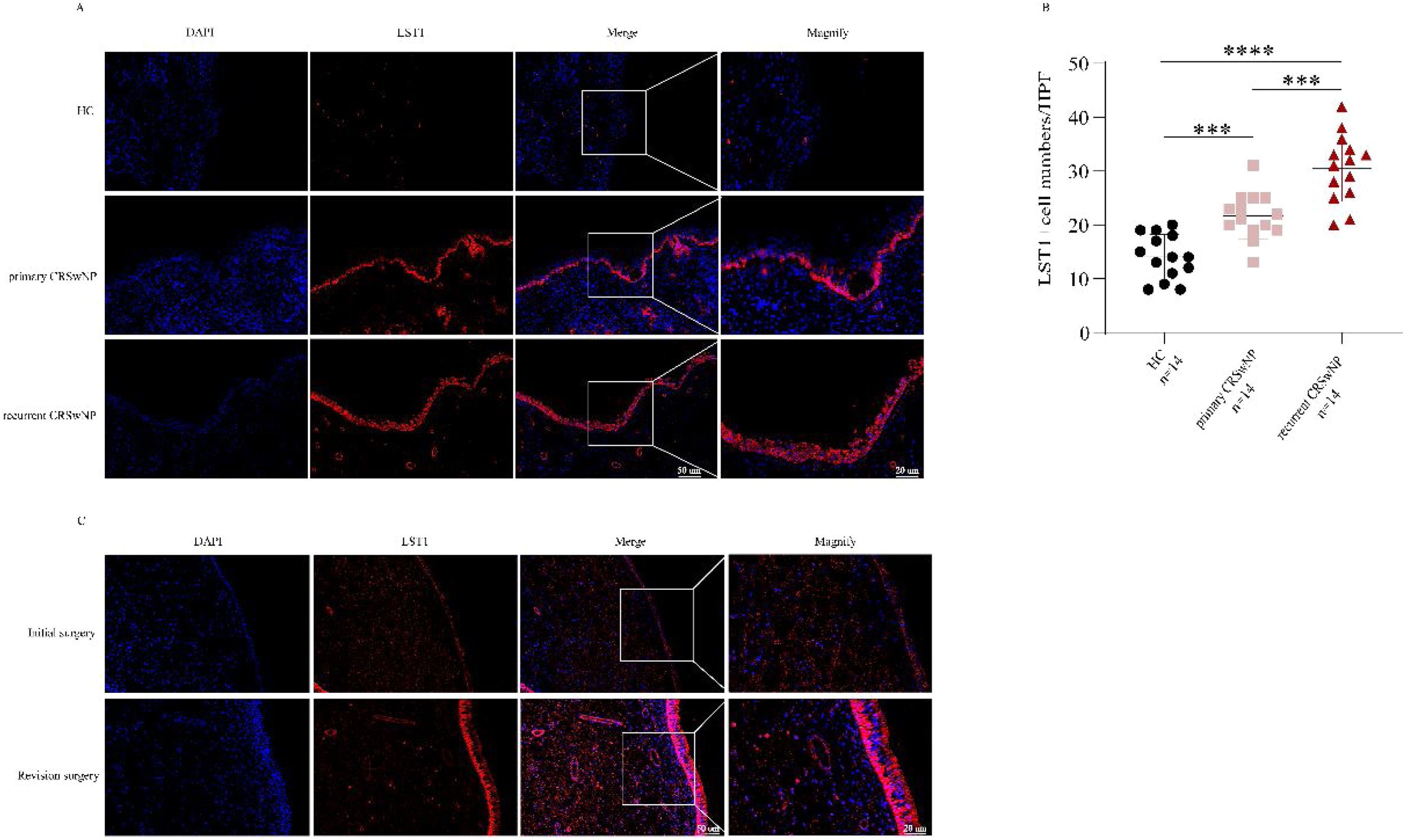
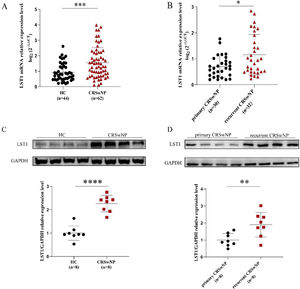
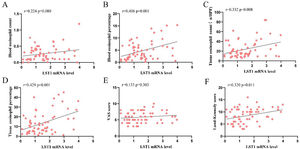
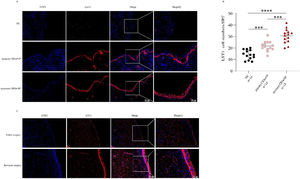
As shown in Fig. 1, the tissue LST1 mRNA levels were clearly elevated in the CRSwNP group than in the HC group. Moreover, compared with the primary CRSwNP group, the LST1 mRNA levels were significantly increased in the recurrent CRSwNP group. Furthermore, WB results presented that the protein expression levels of LST1 were enhanced in the CRSwNP group than in the HC group, and this difference was also observed between the primary CRSwNP and recurrent CRSwNP group. Interestingly, the increased tissue LST1 levels were correlated with blood eosinophil percentages (r=0.406, p=0.001), tissue eosinophil counts (r=0.332, p=0.008), and percentages (r=0.429, p<0.001), Lund-Kennedy scores (r=0.320, p=0.011) (Fig. 2). The detailed data were presented in Table 3. In addition, the representative IF images showed that LST1 staining was mainly located in the nasal epithelium and gland areas, and the numbers of LST1 positive cells were significantly greater in the CRSwNP group, especially in the recurrent group than in the HC group, more importantly, we found that the fluorescence intensity of LST1 was significantly enhanced in the re-surgery compared to the initial surgery in the nasal mucosa from the same patient (Fig. 3).
The tissue mRNA expression of LST1 in CRSwNP patients. (A) Compared to the HC group, the tissue LST1 mRNA levels were clearly increased in the CRSwNP group. (B) The tissue LST1 mRNA levels in the recurrent CRSwNP group were significantly higher than in the primary CRSwNP group. (C) WB images of LST1 between the HC group and CRSwNP and its levels were significantly increased in the CRSwNP group than in the HC group. (D) WB images of LST1 between the primary CRSwNP group and recurrent CRSwNP and its levels were significantly increased in the recurrent CRSwNP group than in the primary group. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001. LST1, Leukocyte-Specific Transcript 1; HC, Health Control; CRSwNP, Chronic Rhinosinusitis with Nasal Polyp; WB, Western Blotting.
Increased LST1 mRNA levels correlated with clinical variables in CRSwNP patients. Spearman correlation analysis indicated that tissue LST1 mRNA levels were positively correlated with blood eosinophil percentage (B), tissue eosinophil count (C), percentage (D), and Lund-Kennedy score (F). LST1, Leukocyte-Specific Transcript 1; CRSwNP, Chronic Rhinosinusitis with Nasal Polyp; VAS, Visual Analog Scale.
Correlation between tissue LST1 and clinical variables in CRSwNP patients.
| Variables | r | p-value |
|---|---|---|
| Age, years | −0.043 | 0.737 |
| BMI, kg/m2 | −0.034 | 0.795 |
| Blood eosinophil counts (109/L) | 0.224 | 0.080 |
| Blood eosinophil percentages (%) | 0.406 | 0.001 |
| Tissue eosinophil counts (n/HPF) | 0.332 | 0.008 |
| Tissue eosinophil percentages (%) | 0.429 | < 0.001 |
| VAS scores | 0.133 | 0.303 |
| Lund-Kennedy scores | 0.320 | 0.011 |
CRSwNP, Chronic Rhinosinusitis with Nasal Polyps; BMI, Body Mass Index; VAS, Visual Analogue Scale.
IF expression of LST1 in the tissue among three groups. (A) Representative IF images of HC, primary CRSwNP and recurrent CRSwNP (magnification ×200, ×400). (B) The LST1+ cell numbers were significantly higher in the CRSwNP group, especially in the recurrent CRSwNP group than in the HC group. (C) IF images of LST1 in initial surgery and re-surgery tissues from the same CRSwNP patient. ***p<0.001; ****p<0.0001. LST1, Leukocyte-Specific Transcript 1; HC, Health Control; CRSwNP, Chronic Rhinosinusitis with Nasal Polyp. IF, Immunofluorescence.
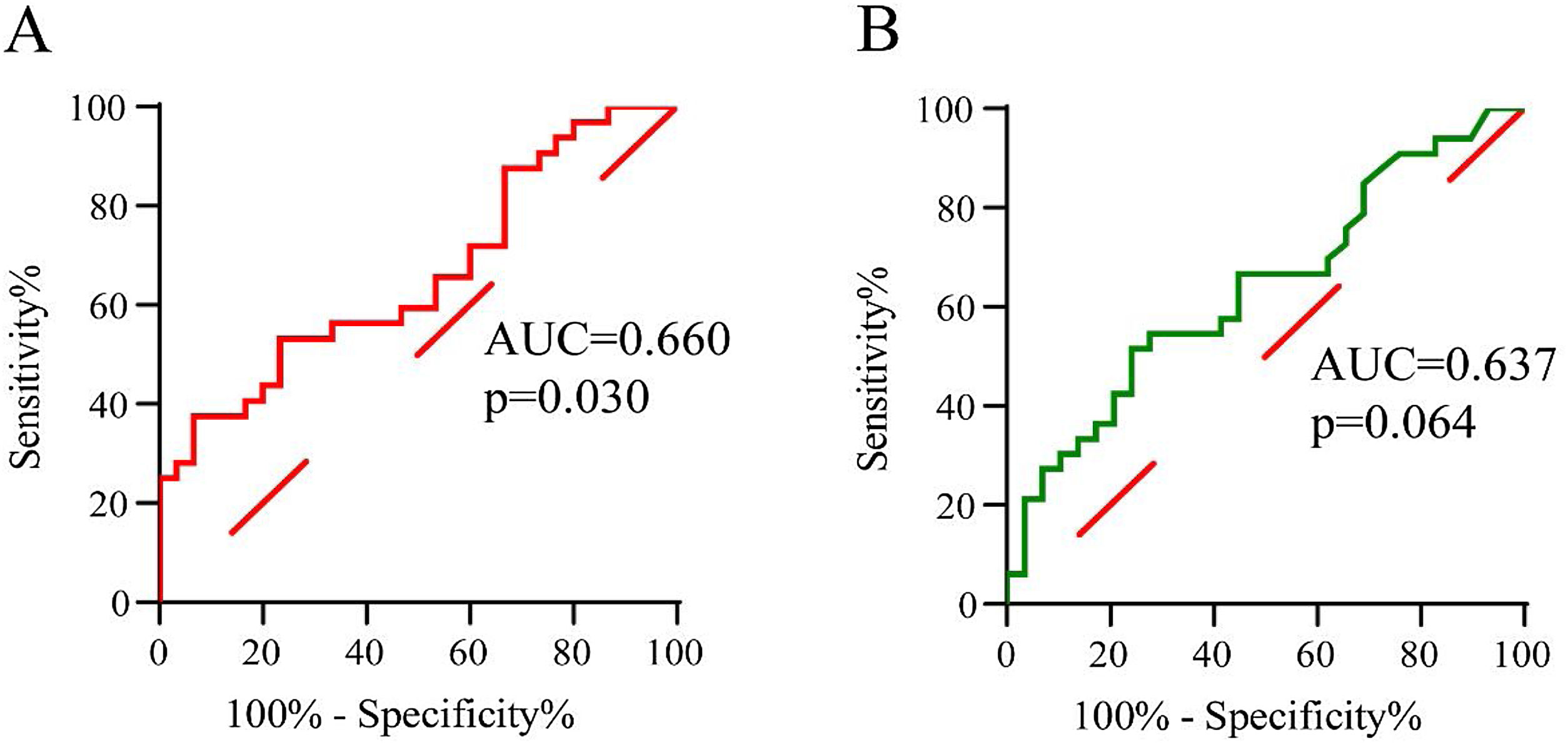
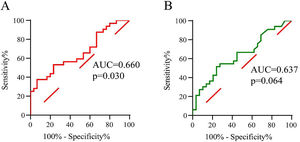
To evaluate the predictive value of tissue LST1 mRNA levels for CRSwNP postoperative recurrence, the ROC curves analysis was performed (Fig. 4). The results demonstrated that tissue LST1 mRNA levels (AUC=0.660, p=0.030) exhibited better predictive ability for CRSwNP recurrent than tissue eosinophil percentages (AUC=0.637, p=0.064). The detailed data were displayed in Table 4.
ROC analysis results of different variables for predicting recurrence of CRSwNP.
| Variables | AUC | p-value | 95% CI | Sensitivity | Specificity | Cut-off value |
|---|---|---|---|---|---|---|
| Tissue eosinophil percentages | 0.637 | 0.064 | 0.500‒0.775 | 0.515 | 0.759 | 13.5 |
| Tissue LST1 mRNA levels | 0.660 | 0.030 | 0.525‒0.796 | 0.375 | 0.933 | 1.2 |
ROC, receiver operating characteristic; AUC, the area under the curve; CI, confidence.
In this study, our results demonstrated that the tissue LST1 mRNA expression levels were increased in the CRSwNP group than in the HC group and the elevated levels were associated with peripheral eosinophil percentages, tissue eosinophil counts and percentages. Moreover, IF staining and WB results showed that LST1 was highly expressed in the CRSwNP group, especially in the recurrent CRSwNP group, which might involve in the course of CRSwNP and associate with the disease recurrence. Furthermore, ROC curves indicated that tissue mRNA levels of LST1 have a better value for predicting CRSwNP recurrence. Taken together, these results suggested that tissue LST1 might involve in the occurrence and development of CRSwNP and its levels could be a crucial biomarker for predicting recidivation in CRSwNP patients.
LST1, as a pro-inflammatory factor, is mainly expressed in DCs and other immune cells,10,11,18 and LST1 overexpression plays an important role in DCs function by inducing the formation of filopodia based on previous studies.18–20 A series of the literature suggested that DCs were the most potent and specialized antigen-presenting cells and could stimulate T cells differentiation into Th2 cells, which were essential for the Th2 immune responses and eosinophil movement.21,22 Moreover, prior data indicated that DCs were involved in the occurrence and development of CRSwNP by inducing eosinophil infiltration into the tissues.23,24 It is well known that CRSwNP is Th2 cytokines mediated chronic inflammatory response, and the tissue abundance of eosinophilic infiltration acted as a main pathological feature of CRSwNP, according to previous studies.1,25 Therefore, we hypothesized that LST1 might involve in the occurrence and development of CRSwNP by promoting Th2 cells differentiation and contributing to eosinophil infiltration in the nasal mucosa. In the present study, we found that tissue LST1 mRNA expression levels were higher in the CRSwNP group than in the HC group, and its elevated levels were positively correlated with peripheral eosinophil percentages and tissue eosinophil counts and percentages. Furthermore, IF staining and WB results showed that LST1 protein expression levels were significantly elevated in the CRSwNP group compared with the HC group. Thereby, it was reasonable to suggest that LST1 was involved in the pathogenesis of CRSwNP and was closely relative to the eosinophilic inflammation of nasal mucosa. However, in the current study, we were unable to determine a causal relationship between LST1 expression and tissue inflammation. Although previous studies found that LST1 expression was correlated with immune cell function, the potential role of LST1 in CRSwNP and its relationship with inflammation is still unclear. Therefore, the mechanism of this potential involvement needs to be further explored.
Up to date, although Function Endoscopic Sinus Surgery (FESS) is conducive to improve postoperative quality of life and prognosis in CRSwNP patients, recurrence still occurs in some patients.26,27 Previous publications indicated that the degree of local eosinophil infiltration in nasal polyp tissue was closely concerned with the disease recurrence.28,29 Van et al.30 described that patients with elevated eosinophil counts in nasal biopsy specimens were more likely to require surgery again. In this study, we found that tissue eosinophil counts and percentages in the recurrent CRSwNP group were higher than in the primary CRSwNP group. Moreover, the tissue LST1 levels were notably raised in the recurrent CRSwNP group compared with the primary CRSwNP group, and its increased levels were tightly associated with tissue eosinophils counts and percentages. In addition, IF staining and WB results showed that the LST1 protein expression levels were significantly elevated in the recurrent CRSwNP than in the primary CRSwNP group. Furthermore, ROC curves showed that tissue LST1 mRNA levels have a better ability in predicting recurrent CRSwNP. Given that, we hypothesized that higher levels of LST1 in the nasal mucosa might aggravate the Th2 inflammatory response and promote the recruitment of eosinophils, resulting in poor prognosis and a higher risk of recurrence in CRSwNP. Thus, we deemed that tissue LST1 mRNA levels might be used as an objective indicator to predict postoperative recurrence in patients with CRSwNP.
However, our study had some limitations. First, there was no unified criteria criterion regarding the diagnostic criteria of recurrent CRSwNP, which might limit the applicability of our findings. Second, we only measured the tissue levels of LST1, and its concentration in circulating and nasal lavage fluid was not detected. Third, the sample size was relatively small, and the participants were recruited from a single medical center, which might increase the risk of selection bias. Lastly, certain confounding factors also have an impact on the prognosis of CRSwNP, such as treatment adherence, the surgical technique used, and postoperative care, which were extremely important for a good prognosis of CRSwNP. Thereby, a prospective study with a larger sample size and extended follow-up period while actively controlling for the effects of confounding factors is warranted to confirm the findings of the present study and evaluate more accurate predictive values for LST1 mRNA expression in the future.
ConclusionThe study results indicated that tissue LST1 mRNA expression levels were up-regulated in CRSwNP and associated with mucosa eosinophilia infiltration. Statistical analyses showed that tissue LST1 might be a potential candidate biomarker for predicting CRSwNP recurrence. These findings might contribute to understanding the underlying pathogenesis of recurrent CRSwNP, which could provide a novel and effective intervention target and improve precise treatment.
Ethics approval and consent to participateThis study was conducted in accordance with the recommendations of Declaration of Helsinki. The Human Ethical Committee of Xiangya Hospital of Central South University approved this study. All participants provided informed consent.
Conflicts of interestThe authors declare no conflicts of interest.
This work was supported by the National Natural Science Foundation of China (No. 82171118, No. 81800917, and No. 81873695) and the Natural Science Foundation of Hunan Province (No. 2020JJ4910).