Basaloid squamous cell carcinoma (BSCC) is a rare subtype of squamous cell carcinoma (SCC). Because of its rarity, both clinical and prognostic features of this variant are not well known.
ObjectiveIn this study, we aimed to determine the frequency of BSCC and other SCC variants among all laryngeal SCC cases, and to determine clinical and prognostic features of BSCC variant. Study design: retrospective cohort study. Evidence level: Level 2b.
Material and methodsRecords of the patients who had laryngeal SCC surgically treated at our institute between 2007 and 2013 were retrospectively reviewed.
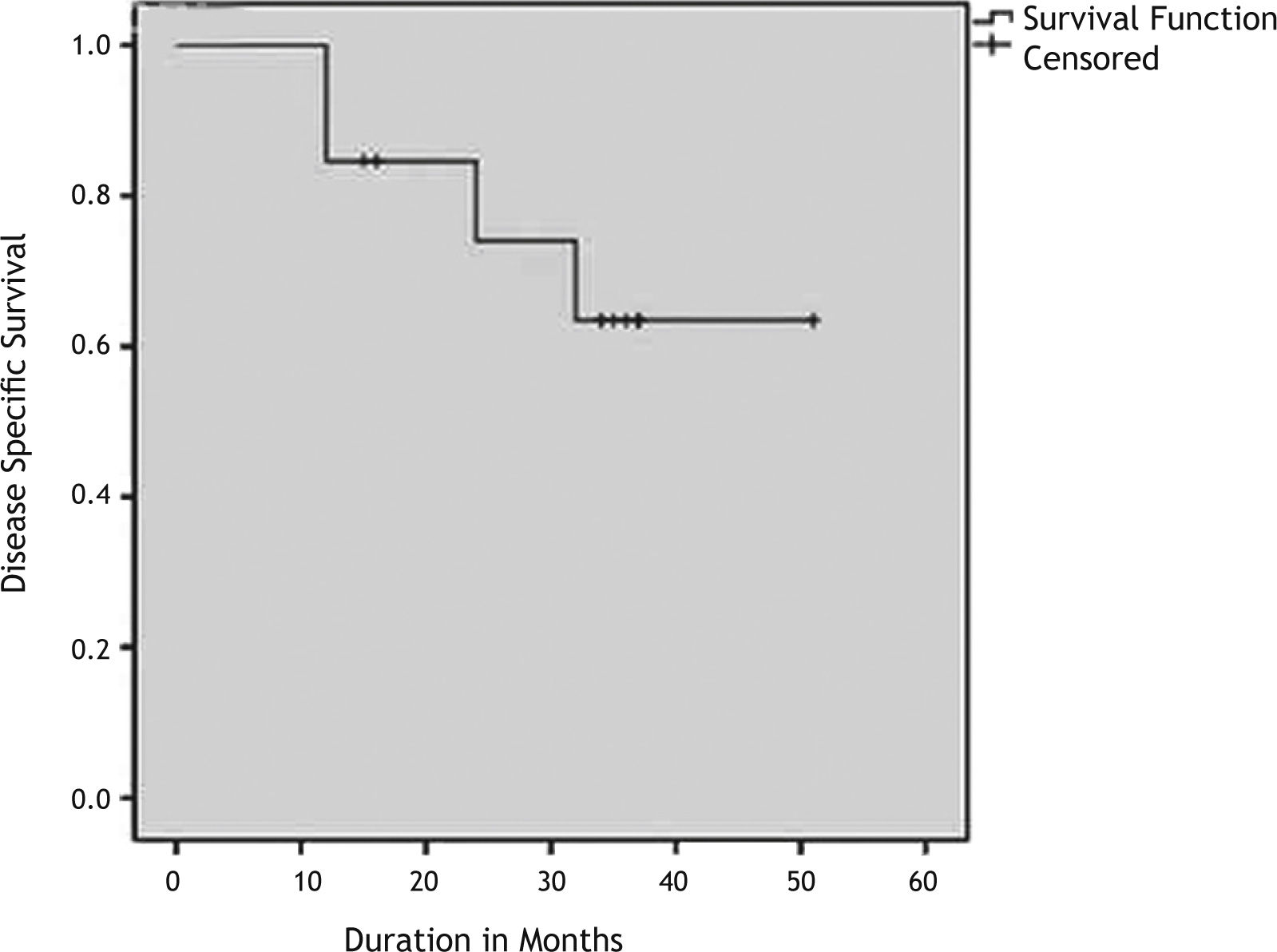
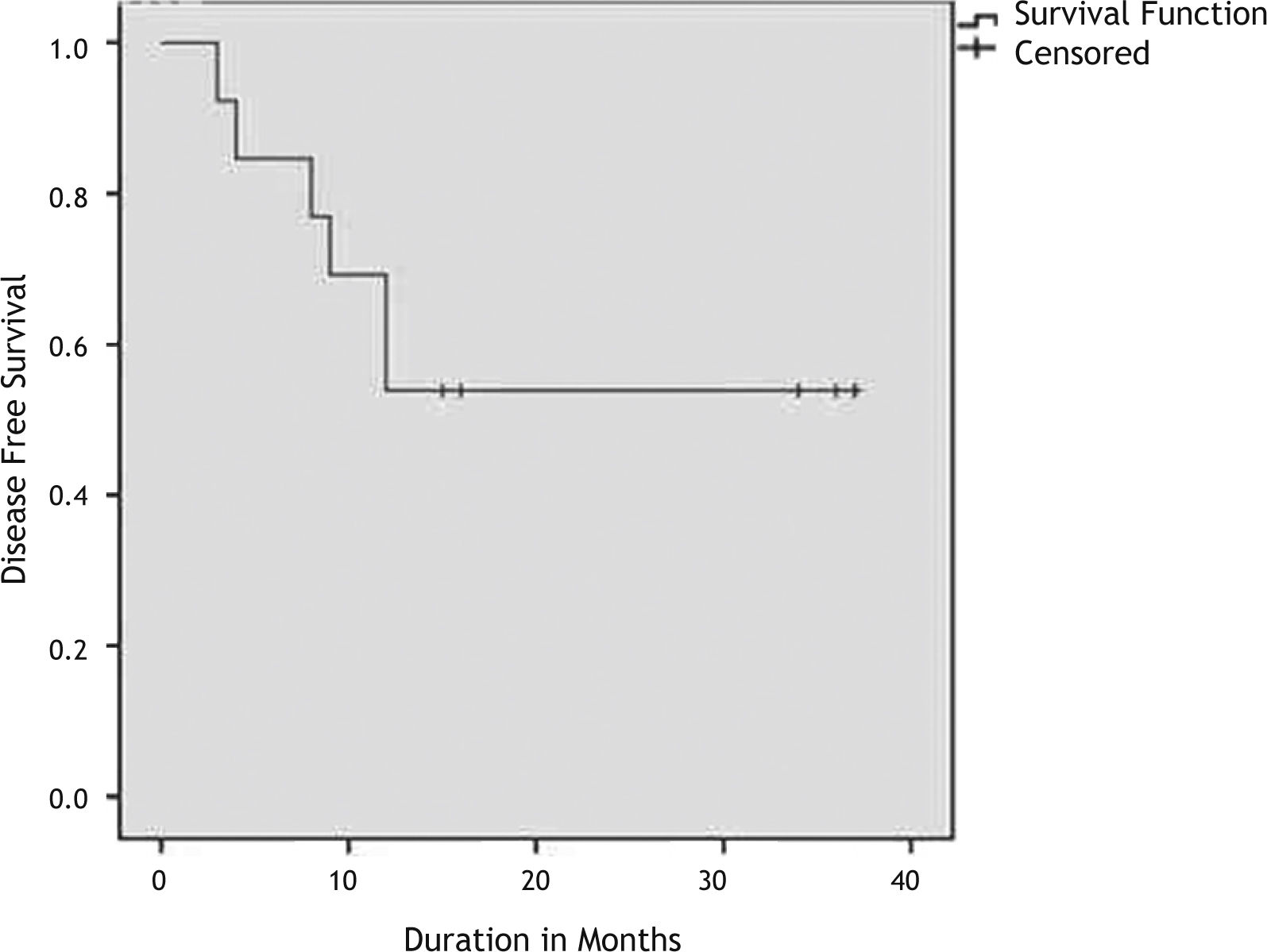
ResultsAmong 198 subjects who had laryngeal SCC surgically treated, the frequency of the variants of SCC other than classical variant was 10.1% (20/198). The most common SCC variant was BSCC (6.6%). Eleven (84.6%) patients with BSCC were at an advanced stage at the presentation (p>0.05). The 3-year overall survival and disease-free survival rates were 63% and 53% respectively.
ConclusionBSCC variant may be more common than previously reported. Since almost the half of patients experiences disease recurrence in the early period, multimodal treatment strategies should be employed at initial treatment, and a close follow-up is strongly recommended for this aggressive SCC variant.
O carcinoma escamoso basaloide (CEB) é um raro subtipo do carcinoma de célula escamosa (CCE). Em decorrência de sua raridade, os aspectos clínicos e prognósticos dessa variante não são bem conhecidos.
ObjetivoDeterminar a frequência de CEB e de outras variantes do CCE entre todos os casos de CCE da laringe, assim como os aspectos clínicos e prognósticos da variante CEB.
Materiais e métodosTrata-se de um estudo de coorte retrospectivo. Nível de evidência: 2b Os registros dos pacientes tratados cirurgicamente para CCE de laringe em nossa instituição entre 2007 e 2013 foram retrospectivamente revisados.
ResultadosForam anotados 198 pacientes tratados cirurgicamente para CCE de laringe. A frequência das variantes de CCE diferentes da variante clássica foi 10,1% (20/198). A variante de CCE mais comum foi CEB (6,6%). Por ocasião da apresentação inicial, 11 (84,6%) pacientes com CEB estavam em estágio avançado (p>0,05). Os percentuais de sobrevida geral após três anos e de sobrevida livre da doença foram 63% e 53%, respectivamente.
ConclusãoA variante CEB pode ser mais comum do que o informado anteriormente. Considerando que praticamente metade dos pacientes sofre recorrência da doença em seu período inicial, devem ser introduzidas estratégias terapêuticas multimodais no tratamento inicial; além disso, recomendamos enfaticamente um cuidadoso seguimento para essa agressiva variante do CCE.
Squamous cell carcinoma (SCC) is the most common malignancy of the upper aerodigestive tract (UADT), and basaloid variant is a rare subtype. In 1986, BSCC was first described by Wain et al. as a distinctive SCC variant with an aggressive behavior.1 In the head and neck region, the most common sites of involvement for BSCC are epiglottis, piriform sinus and base of the tongue.2,3 Other less common sites of origin are oral cavity, tonsils, sinonasal tract, nasopharynx, and trachea.2,3 Since the first cases were described by Wain et al., approximately a hundred of cases of BSCC have been reported at laryngeal location in a 25-year period.4According to previous reports, these tumors often present at an advanced T (tumor) stage.4 Another characteristic of this histological variant is the presence of common regional metastases at its presentation.4 In addition, distant metastases are not rare with this histological subtype, and they result in aggressive behavior and poor prognosis, which is characteristic of this type of tumor, according to most authors.1–4
In this report, we aimed to determine the frequency of BSCC and other SCC variants among all laryngeal SCC cases, and to determine clinical and prognostic features of BSCC variant.
Materials and methodsThe development of this study took place in complete agreement with the Declaration of Helsinki and with the International Conference on Harmonization/Good Clinical Practice guidelines and it was approved by the local ethics committee. Records of the patients who had laryngeal SCC surgically treated at our institute between 2007 and 2013 were reviewed retrospectively. Since distinction of basaloid variant and other variants could be difficult or erroneous in small specimens, patients who were only subjected to biopsy, and treated with radiation/chemoradiation were excluded. Subjects who had any malignancy other than laryngeal SCC were also excluded. According to the tumor stage, to the patient and to the tumor variables, the study patients had laser/laryngofissure cordectomy, partial laryngectomy, total laryngectomy or laryngopharyngectomy. The diagnosis of BSCC was made according to the criteria defined by Wain et al.1 Clinical data regarding age, gender, alcohol and tobacco use, stage, treatment, and follow-up were reviewed. Clinical and pathological TNM staging of the tumors were done appropriately according to the 6th and 7th editions of AJCC Cancer Staging Manual. The surveillance was performed according to the NCCN guidelines.
Statistical analysis were performed with SPSS Statistics 20.0 package. Kaplan-Meier survival analysis was performed to evaluate overall survival (OS), disease specific survival (DSS) and disease free survival (DFS). Data regarding disease status in the end of the follow-up were entered as “no evidence of disease”, “alive with disease”, “died from disease”. The “event” in Kaplan Meier survival analysis was “death” for the calculation of overall survival, “death due to disease” for the estimation of disease specific survival and “local or systemic recurrence of the disease” for the calculation of the disease free survival. One of the cases (subject #1) has been lost to follow-up and included in the case list as the worst outcome. Fisher's exact test was used to evaluate the difference of advanced stage presentations between BSCC and classical SCC variant subjects. Statistical significance of p<0.05 was taken as criterion.
ResultsThere were 421 patients diagnosed and treated with laryngeal cancer between 2007 and 2013. The numbers of patients were 115, 136, 89 and 81 on stage I, II, III and IV respectively. Among those 421 patients, 198 subjects underwent surgical treatment during the review period. Twenty (10.1%) of them subjects had SCC variants (Table 1), among those, 13 (6.6%) had the basaloid variant of SCC. The average age was 63.2±9.8 (range: 44-77) years, and all subjects were males.
All of the subjects had smoking history, but none had history of chronic alcohol use. The demographic data, including age, clinical and pathological TNM classifications and stages of the subjects are presented in Table 2. Two subjects (15%) had early stage (Stage I/II) laryngeal cancer, while 11 (85%) had advanced stage (Stage III/IV). In contrast, among 178 ordinary laryngeal SCC subjects surgically treated in the same period, 30% were on early stage, and 70% were on advanced stage laryngeal cancer. The rates of cases on advanced stage at the detection are not significantly different among basaloid and classical SCC groups (p=0.353)
Clinical and prognostic data of the studied subjects.
| Case | Age (years) | Location | Surgical Procedure | LV inv | PN inv | Regional metastases | Metastatic Nodes | cTNM | pTNM | Stage | Follow-up (months) | Local/Regional recurrence | Distant metastase |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 44 | SG | SL+Bil SND (zones 2,3,4) | – | – | – | – | T2N0 | T2N0 | II | LFU | None | None |
| 2 | 75 | GL | Left MRND (CN IX), 4 months later TL+ Right Hemithyroidectomy+Right SND (zones 2,3,4) | – | – | + | One lymph node in ipsilateral zone 2, three lymph nodes in ipsilateral zone 4 andfive lymph nodes in ipsilateral zone 5 | T4aN0 | T4aN0 | IVA | 32, DOD | Peristomal recurrence after seven month | None |
| 3 | 51 | TG | TL+Bil SND (zones 2,3,4) | – | – | – | – | T4aN0 | T4aN0 | IVA | 51, AWD | Metestatic lymph node in zone 1 after nine months/Treated with neck dissection | None |
| 4 | 71 | SG | TL+Right MRND (CN IX),+Left SND (zones 2,3,4) | – | – | + | One lymph node in each zone 3 | T3N2c | T3N2c | IVA | 37, AWD | None | None |
| 5 | 77 | GL | TL+Left MRND (CN IX), | – | – | – | – | T4aN0 | T4aN0 | IVA | 37, AWD | None | None |
| 6 | 63 | GL | TL+Bil SND (zones 2,3,4) | – | – | – | – | T4aN0 | T2N0 | II | 36, AWD | None | None |
| 7 | 63 | TG | TL+Left MRND (CN IX) | + | + | + | One lymph node in ipsilateral zone 4 | T4aN1 | T4aN1 | IVA | 35, AWD | None | None |
| 8 | 62 | SG | TL+Bil SND (zones 2,3,4) | – | – | – | – | T3N0 | T3N0 | III | 34, AWD | None | None |
| 9 | 76 | GL | TL+Bil SND (zones 2,3,4) | – | – | – | – | T4aN0 | T4aN0 | IVA | 24, DOD | Peristomal recurrence after 12 months | None |
| 10 | 63 | GL | TL+Total Thyroidectomy+Bil SND (zones 2,3,4)+Zone 6 dissection | – | – | – | – | T4aN0 | T4aN0 | IVA | 12, DOD | None | Lung metestasis after three months |
| 11 | 59 | TG | TL+Right MRND (CN IX),+Left SND (zones 2,3,4)+Right Hemithyroidectomy | + | + | – | – | rT3N0 | T3N0 | III | 16, AWR | None | Lung metestasis after eight months |
| 12 | 61 | SG | SL+Bil SND (zones 2,3,4) | – | – | – | – | T2N0 | T4aN0 | IVA | 16, AWD | None | None |
| 13 | 58 | SG | TL+Bil SND (zones 2,3,4) | – | – | – | – | rT3N2c | T4aN0 | IVA | 15, AWD | None | None |
LV inv, lymphovascular; PN inv, perineural invasion; ART, Adjuvant Radiation Therapy; DOD, Died Of Disease; AWD, Alive Without Disease; AWR, Alive with Reccurence; SG, Supraglottic; GL, Glottic; TG, Transglottic; FND, Functional Neck Dissection; CN, Cranial Nerve; Bil, Bilateral; MRND, Modified Radical Neck Dissection.
All subjects were diagnosed with laryngeal cancer at presentation except for the subject #2. This subject was presented with a neck mass of unknown primary and underwent neck dissection at first and had radiotherapy. He had total laryngectomy four months after primary treatment due to a late presenting glottic tumor as primary focus.
The tumors were glottic, supraglottic, and transglottic in five (38.5%), five (38.5%), and three (23.0%) subjects respectively. One subject (Subject #1) was lost to follow-up (LTF) after 12 months. The average follow-up time was 28.7±11.9 (range, 15-51) months (LTF subject was excluded). At the time of diagnosis, two subjects (Subjects #4 and 7) were N+ (Table 2). The operations performed are also summarized in Table 2. The most common operation was total laryngectomy (84.6%) due to advanced T stages.
Definitive diagnosis of BSCC was made after examination of the main surgical specimen. The punch biopsy investigations before the main surgery had correctly identified BSCC in only two cases, however two cases were erroneously identified as papillary variant and the remaining nine as the classical variant. The average of the largest tumor diameter was 3.05±1.12cm (range, 2-5cm) in the BSCC patients. None of the patients with BSCC have HPV positivity.
Postoperative complications developed in two subjects: one pharyngocutaneous fistula (subject #10) and one chylous fistula (subject #13). Both were successfully treated with conservative therapy.
Recurrence was observed in 5 subjects (38%). Loco-regional recurrence developed in three subjects and distant metastases were seen in two subjects (Table 2).
Two subjects (subjects #11 and #13) had previous treatment for laryngeal carcinoma. Subject #13 had concomitant chemo-radiotherapy and recurrence seven months after the initial treatment. Subject #11 had radiotherapy for early-stage supraglottic larynx carcinoma and experienced recurrence nine months after the initial treatment.
All patients who have died were dead because of the disease so overall survival and disease specific survival times were the same. The Kaplan Meier survival curves depicting the DSS and DFS are shown in Figs. 1 and 2. The 3-years OS rate was 63%. The 3-years DFS rate was 53%. The 3-years OS rate and DFS rate for the patients with other squamous cell carcinomas who has undergone surgery was 77% and 61% respectively.
DiscussionHistopathologically, the BSCC is characterized by nests of basal-type squamous tumoral cells that classically have central necrosis. In a certain small biopsy specimen, when this classical morphology is not observed, the initial pathological diagnosis would be an ordinary SCC of varying grade. Because this subtype may be associated with a relatively worse prognosis, the biopsy diagnosis can be considered to have more importance. In our series of 13 histopathologically-proven cases, the final diagnoses for 11 patients were established in the resection specimens. Only 2 cases were diagnosed as basaloid type SCC in the initial endoscopic biopsy samples. The most credible explanation for this discrepancy between the biopsy and resection samples is that a basaloid or papillary SCC quite expectedly might contain foci of ordinary SCC morphology and unless the initial biopsy has areas representing the characteristic morphology for the diagnosis of a specific subtype, a small sample can be misleading. Moreover, many SCCs have superficial papillary architecture that might lead to a papillary SCC diagnosis in the biopsy sample. For an SCC to be diagnosed as a papillary type SCC, the excision or resection specimen must preferably be examined; which can present a morphology consisting of an SCC that predominantly has prominent papillary growth without a dominant SCC component that is invading underneath. Our series and results clearly indicate the inevitably-limited representation of the initial biopsy for the diagnosis of the correct subtype.
The most common laryngeal malignancy is SCC, which comprises more than 90% of the cases. Verrucous, spindle cell and basaloid subtypes are much rarer variants. BSCC first described in 1986 and has been reported in larynx, hypopharynx, oral cavity, and oropharynx at first.1,3–5 Approximately one fourth of the cases reported within the UADT are located at the larynx and these cases comprise only less than 1% of the laryngeal cancers.4 In our series, 10% of the surgically treated laryngeal carcinomas were other than classical variants of SCC and 65% of all these variants were BSCC. This is the first study, which reports frequency of SCC variants located at the larynx and also systematically describes the clinical and prognostic features. Relatively high incidence of BSCC in our series may be related with higher awareness of pathologists at our institution about SCC variants.
BSCC displays distinct morphological and biological features and have a different clinical course.6 According to previous reports, it mainly affects elderly men who have smoking/alcohol abuse history as classical SCC.3,5,6 Ferlito et al. reported that the average age of the patients who have BSCC located at larynx or hypopharynx is 63 years.5 Similar to the specialized literature, all the patients have smoking history and the average age at diagnosis was 63.2 years in our series.
There are some studies that reported predilection for the supraglottic subsite.4,7 In the present study, the frequencies of supraglottic and glottic involvement were equal which is contradictory to those previous reports. The patients with BSCC usually present at more advanced stages (stage III&IV).4 In our series, 85% of the patients were at advanced stages. But the rate of BSCC with advanced stage at presentation is not significantly different from classical variant SCC group according to our results.
T1, T2 early laryngeal tumors are managed by either primary radiotherapy or surgery (laser cordectomy or laryngofissure with cordectomy). Advanced stage tumors are managed by either organ preserving protocols (concurrent radiotherapy and chemotherapy) or surgery (with or without postoperative radiotherapy) according to patients age, pulmonary status and preferences. According to our findings, the initial biopsy showed the BSCC variant in only 2 of the 13 patients diagnosed as BSCC. Therefore as only 15% of the patients can be diagnosed with the initial biopsy, the diagnosis should always be deferred to final pathology. Currently the surgery is the preferred primary treatment modality for BSCC in our institution. For that reason, we could not present data regarding the possible value of other treatment options for BSCC. Future controlled studies comparing the effectiveness of surgery versus other modalities such as chemoradiotherapy and/or radiotherapy may clarify this issue.
To date, there are no clinical studies proposing a specific therapeutic strategy for management of BSCC. However surgery is the mainstay for the treatment of BSCC of the larynx due to advanced stage of the patients at presentation.4 Total laryngectomy is the most commonly performed surgical procedure in the specialized literature.4 In our series, most of the patients were treated with surgery with postoperative radiotherapy (92%). We performed total laryngectomy in 85% of the patients. However, the type of laryngeal surgery and the extent of neck dissection vary according to the stage and metastatic pattern of the disease. Soriano et al. suggested an organ preservation approach with induction chemotherapy by considering the high risk of distant metastases and the higher morbidity of surgery.8 This strategy should be taken with caution since the number of reported patients was limited. But these patients might be good candidates for regimens incorporating chemotherapy and radiotherapy after surgery since majority of them present with advanced stages and many develop distant metastasis.
It is generally believed that BSCC has a worse prognosis than the classical form of SCC but there are also some contradictory reports.1,9–11 Three studies compared BSCCs with stage matched SCC controls. Wizenburg et al. found that the 2-year survival rates were 23.5% and 53% for BSCCs and SCCs respectively.12 In a study by Erdamar et al., 3-year survival rates were 50% and 72% for BSCC and SCC groups.7 These two studies reports survival rates of BSCC cases located at different parts of UADT. In contrast, Luna et al. compared 6 BSCC cases with 47 classical variant SCC cases of the pyriform sinus and found no survival difference.11 The authors concluded that survival rates would be similar to that of conventional SCC when the anatomical site, clinical stage and treatment were matched.
The overall survival rate and disease free survival rate for the patients with other squamous cell carcinomas who has undergone surgery was 77% and 61% respectively. This figure was much better than the overall survival rate and disease free survival rate for the patients with BSCC 63% and 53% respectively. Meanwhile the BSCC tumors present at more advanced stage compared to other squamous cell carcinomas. Therefore treatment of BSCC variant involves more aggressive therapy than other squamous cell carcinomas. Nevertheless despite more aggressive therapy, the survival was worse for BSCC compared to that with other squamous cell carcinomas treated surgically. In our series, an aggressive behavior of the BSCC cases was observed. There were four highly mortal events within a year: two stomal recurrences (15.4%) and two distant metastases (15.4%). Compared to the specialized literature, these rates are higher. Stomal recurrence following primary total laryngectomy for squamous cell carcinoma occurs in 2%–6%.13,14 The incidence of distant metastasis varies according to the site of the primary tumor: 3.1% to 8.8% in glottic SCC, and 3.7% to 15% in supraglottic SCC.15
ConclusionAccording to our series, BSCC of the larynx may not be as rare as previously reported. As the awareness of the patholologists increases, the true incidence may be revealed. Aggressive behavior in the form of stomal recurrence or distant metastases within the first year of the postoperative period is as high as 30%. Three-year DFS was 53% in our series, and this rate is lower when compared to the previous reports regarding laryngeal SCC cases.8 Another characteristic of this SCC variant is advanced stage at presentation. As a result multimodal treatment strategies would be employed at initial treatment and close follow-up is strongly recommended according to our experience.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Tutar H, Aydil U, Ekinci O, Bakkal FK, Tutar VB, Kizil Y, et al. The basaloid variant of squamous cell carcinoma of the larynx. Braz J Otorhinolaryngol. 2014;80:245-50.