We aimed to evaluate the effect of radiofrequency turbinate reduction as an initial treatment on clinical improvement, inflammatory mediators, and remodeling process.
MethodsBetween July 2018–February 2020, 32 patients with moderate-severe persistent AR were randomly divided into 2 groups. Intervention group received radiofrequency turbinate reduction followed by intranasal steroid and Antihistamine H-1 (AH-1), control group received intranasal steroid and AH-1. Both groups were evaluated for clinical improvement (using visual analogue scale based on total nasal symptoms score, peak nasal inspiratory flow, and turbinate size using imageJ) after 4 and 8 weeks of treatment. Inflammatory mediators (ELISA from nasal secretions was performed to measure ECP, IL-5, and HSP-70) and remodeling markers (nasal biopsy followed by immunohistochemistry examination was performed to evaluate MMP-9, TIMP-1, and PAI-1) were evaluated in week 4.
ResultsThree patients dropped out of the study, resulting in 16 patients in intervention group and 13 patients in control group. At week 4, clinical response improved significantly in the intervention group compared to control group (Chi-Square test, p < 0.05). Compared to control, intervention group experienced a reduction of IL-5 and no significant change in ECP level (Mann Whitney test, p > 0.05). Reduction in the ratio of MMP-9/TIMP-1 were significantly higher in intervention group (unpaired t-test, p < 0,05). Meanwhile, increase in HSP-70 in the intervention group was slightly lower than in control group, but the difference with control group was not significant (Mann Whitney test, p > 0.05).
ConclusionEarly radiofrequency turbinate reduction followed by pharmacotherapy given to persistent moderate-severe AR patients give more improvement only in early clinical symptoms and reduce MMP-9/TIMP-1 ratio, thus it might be suggested as one of the adjuvant therapies for the management of moderate-severe persistent AR. However, further investigation with a larger sample size and longer follow-up period is needed.
Level of evidence1B.
Allergic Rhinitis (AR) is a chronic inflammatory nasal disease affecting nearly 10%–30% world population.1 Nasal obstruction is found in 70% of moderate-severe persistent AR and affecting the patients’ quality of life. Moderate-severe persistent AR if left uncontrolled will lead to increased risk of bronchus hyperreactivity or bronchial asthma. The standard management of moderate-severe persistent AR is using intranasal steroid and AH-1. If pharmacology fails to control the symptoms, radiofrequency turbinate reduction can be done. Radiofrequency turbinate reduction is a procedure to reduce the volume of submucosal stroma in inferior turbinate using low energy radiofrequency. It is minimally invasive compared to other turbinate reduction technique, thus can be done with local anesthesia in outpatient clinic. To date, no research has studied the effect of radiofrequency turbinate reduction as an initial treatment prior to pharmacotherapy. It is expected that radiofrequency, given as initial treatment, could control the inflammatory reaction and lead to physiological mucosal remodeling, hence improving the clinical symptoms. This study aims to evaluate the effect of early radiofrequency turbinate reduction on clinical improvement, inflammatory mediators, and remodeling process.
MethodsEthical considerationsThe present study was done between July 2018–February 2020. Ethical approval of the study was obtained from Institutional Ethical Committee (Ethics Approval Letter Number: 0881/UN2.F1/ETIK/2018) as a single blind block randomized clinical trial.
Patient enrollmentThis study was started with 32 patients admitted to our clinic and were diagnosed with moderate-severe persistent AR between July 2018‒February 2020. Diagnosis of AR was made through clinical history, physical examination, and skin puncture test. Patients aged 18–55 years old with moderate-severe persistent AR who came to the outpatient clinic and had signed the informed consent form were included in this study. Moderate-severe AR was defined as having AR symptoms for more than 4 days in a week and more than 4 weeks, with symptoms affecting quality of life. Smokers and patients who presented comorbid diseases such as septum deviation in nasal valve without septal swell body, unilateral inferior turbinate hypertrophy caused by septum deviation, pregnant, severe systemic disease, acute rhinitis or rhinosinusitis within 6 weeks before the study period, nasal polyp, nasal or paranasal tumor, had received other methods of inferior turbinate reduction, posterior nasal neurectomy, functional endoscopic sinus surgery, Caldwell Luc, had received topical steroid for 4 weeks before the study period, and coagulation disorder were excluded. Patients were randomly assigned to two groups of treatment with a single blind block randomization. Intervention group received radiofrequency turbinate reduction followed by intranasal steroid and AH-1, control group received standard treatment, which are intranasal steroid and AH-1.
Treatment planningRadiofrequency turbinate reduction followed by intranasal steroid and AH-1 were given to the patients in intervention group. Local anaesthesia before the procedure was achieved by applying a cotton tamponade soaked in lidocaine adrenaline 1:5000 titration and added with xylocaine gel for 10 min in both nostrils. Then, inferior turbinate in both nostrils were infiltrated by a mixture of 1 mL of lidocaine 2% and 2 mL of sodium chloride 0.9% by 3 mL and 1 mL needles until the inferior turbinates were pale. The radiofrequency probe (made by Sutter, both in monopolar and bipolar modes) was inserted to the distal of inferior turbinate until the black line from the probe was inside the inferior turbinate (approximately 10–12 mm). Radiofrequency turbinate reduction was done for 10 s. Insertion of the probe can be done in 2–3 sites. Patients were observed for 10 min after procedure. If bleeding occurred, anterior tamponade with Netcell® should be applied for 2 × 24 h.
For the pharmacology treatment, patients were treated with intranasal steroid and AH-1 according to ARIA WHO guideline 2008. Fluticasone furoate was given twice a day with two sprays (100 µg) for each nostril for 2 weeks, then was continued for once a day with two sprays (100 µg). Antihistamine H-1 was given 10 mg, once a day. Pharmacology treatment was given for 4 weeks, then intranasal steroid was continued for another 4 weeks.
Sample collection and analysisAfter consent was received, all patients agreed to join the study were being evaluated of their clinical presentation and biomolecular markers as their baseline. Clinical presentation was evaluated through three components, which were clinical symptoms, the degree size of inferior turbinate, and nasal airflow resistance. Clinical symptoms were evaluated by Visual Analogue Scale (VAS) based on Total Nasal Symptoms Score (TNSS). The inferior turbinate was evaluated by using nasoendoscopy and Image J™ program was used to determine the size of the inferior turbinate. Nasal airflow resistance was evaluated using In-Check Nasal Inspiratory Flow Meter by Clement Clarke International ltd England. The examination was repeated for three times with 30 s paused and the highest value was taken.
Inflammatory mediators such as IL-5, ECP, and HSP-70 was examined from nasal secretions. Through nasoendoscopy, Netcell® tampon 3 × 6 cm was placed in inferior meatus for 10 min. Then, it was put inside the transport medium filled with Phosphate Buffered Saline (PBS). Nasal secretions culture medium was centrifuged in 1000xg or 300 rpm for 20 min, then sandwich immunoassay examination was performed directly or the sample could be stored in −20 °C or −80 °C. Enzyme-Linked Immunosorbent Assay (ELISA) spectrophotometry was used to evaluate the amount of IL-5 (Quantikine® ELISA catalogue number D5000B), ECP (My Biosource catalogue number MBS727222), and HSP-70 (My Biosource catalogue number MBS2020836) in both groups.
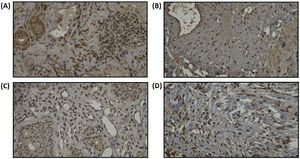
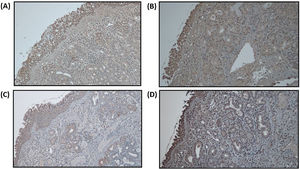
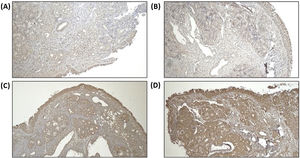
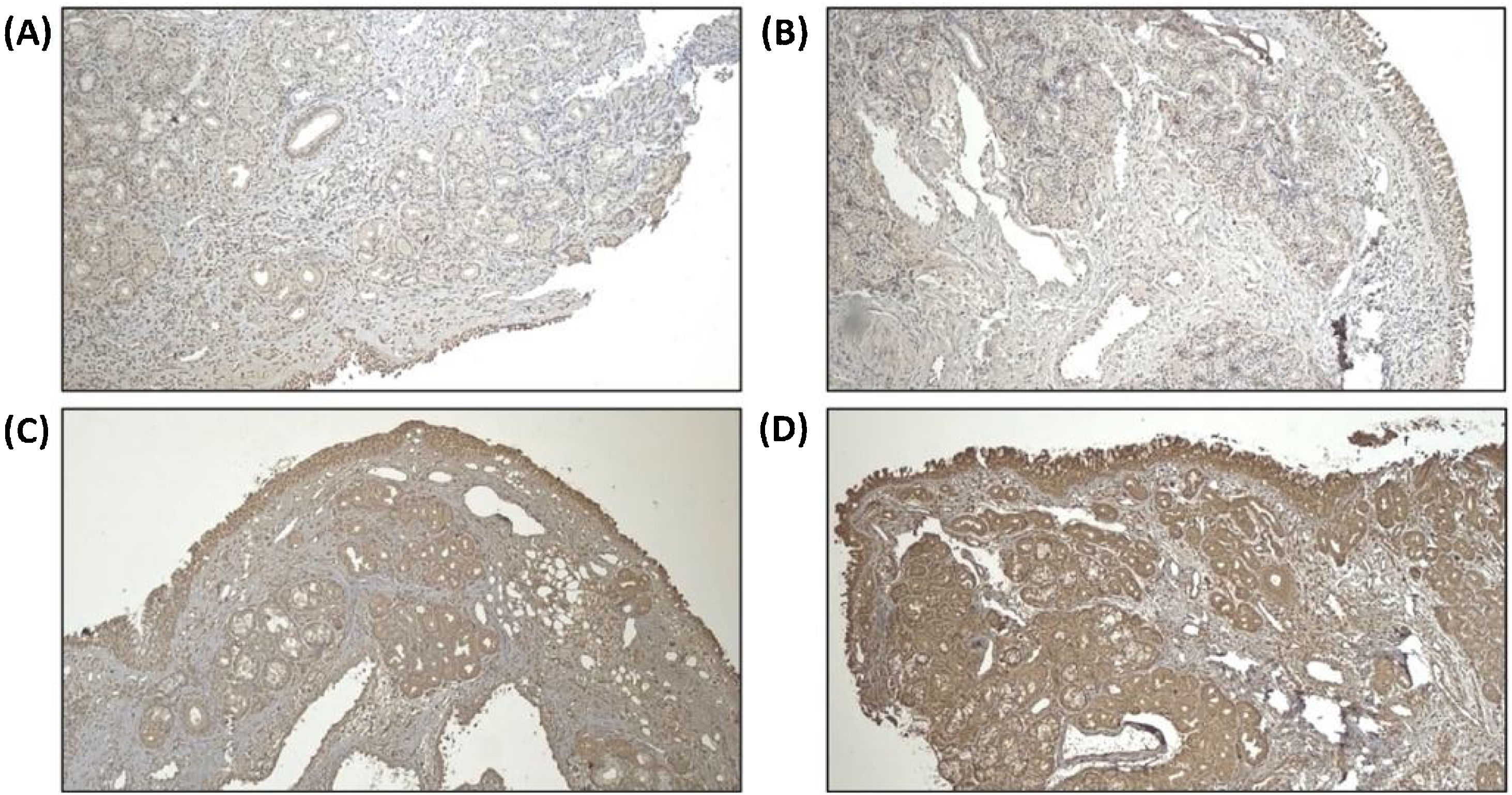
Remodeling components (MMP-9 and TIMP-1) was assessed using immunohistochemistry from tissue biopsy of inferior turbinate. Tissue biopsy was obtained through cunam biopsy from medio inferior region of inferior turbinate. Five mm3 of specimen was obtained for both groups. The specimen obtained from tissue biopsy was put in formalin buffer solution 10% and was sent to Pathology Anatomy Department for immunohistochemistry evaluation. Immunohistochemistry staining using primary antibody from Abcam, Cambridge anti-TIMP-1 Antibody (2A5) ab2464, diluted in 1:100 overnight, anti-MMP-9 antibody (5G3) ab119906, diluted in 1:400 overnight, and anti-PAI1 antibody ab66705, diluted 1:300 overnight.
For MMP-9 and TIMP-1, intensity of immunostaining was assessed by distribution degree according to Can et al. criteria, 1(+) means 25% colored or colored in epithelial surface, 2(+) means 25%–50% colored or colored in epithelial surface, endovascular, perivascular, and vascular basal membrane, 3(+) means 2(+) and 50%–75% colored or colored in inflammatory cells, and 4(+) means 3(+) and 75%–100% colored or colored in matrix.2 For PAI-1 antibody, immunoreactivity index was counted based on positivity and the intensity using Image J™ program. Positivity of immunoreactivity was assessed by counting the amount of positive and negative inflammatory cells using immunohistochemistry staining in 5 field of view with 400× magnification, then the percentage of inflammatory cells positivity was counted by dividing the amount of positive inflammatory cells by total inflammatory cells.
All patients in both groups were being evaluated again of their clinical improvement at 4 weeks and 8 weeks after treatment. Inflammatory mediators and remodeling markers (nasal biopsy followed by immunohistochemistry examination was performed to evaluate MMP-9, TIMP-1, and PAI-1) were evaluated in week 4.
Statistical analysisMedian, lowest, highest, mean, standard deviation, frequency, and ratio values were used as the descriptive statistics of the data. Distributions of the variables were evaluated with Kolmogorov-Smirnov test. Independent t-test or Mann-Whitney test were used in the analysis of two independent quantitative groups. Paired t-test or Wilcoxon test were used in the analysis of two paired quantitative groups. Chi-Square test was used to analyses independent qualitative data. Mc Nemar test was used in the analysis of pre and post treatment within each group. SPSS 25.0 for Mac program was used to perform the statistical analysis.
ResultsThe consort flow diagram is presented in Fig. 1. Three patients from the control group (9.38% dropped out rate) were dropped out, two subjects were loss to follow up, one rejected biopsy in week 4 after treatment. The mean age of patients in this study was 26.9 ± 8.3 and 35.0 ± 12.3 for intervention and control group respectively. No significant difference was found in all baseline characteristics of the patients among the two groups (p > 0.05), except for gender (p < 0.05) (Table 1).
Patients’ characteristics data.
| Patients’ characteristics | Intervention group (n = 16) | Control group (n = 13) |
|---|---|---|
| Gender, n (%) | ||
| Male | 7 (43.8) | 1 (7.7) |
| Female | 9 (56.2) | 12 (92.3) |
| Age, mean ± SD | 26.9 ± 8.3 | 35.0 ± 12.3 |
| Education, n | ||
| Elementary | 0 | 1 |
| Junior High School | 1 | 1 |
| Senior High School | 9 | 7 |
| Bachelor | 6 | 4 |
| History of allergy in family, n (%) | ||
| Present | 7 (43.8) | 4 (30.8) |
| Absent | 9 (56.3) | 9 (69.2) |
| Atopic Dermatitis, n (%) | ||
| Present | 8 (50) | 4 (30.8) |
| Absent | 8 (50) | 9 (68.2) |
| Asthma, n (%) | ||
| Present | 6 (37.5) | 1 (7.7) |
| Absent | 10 (62.5) | 12 (92.3) |
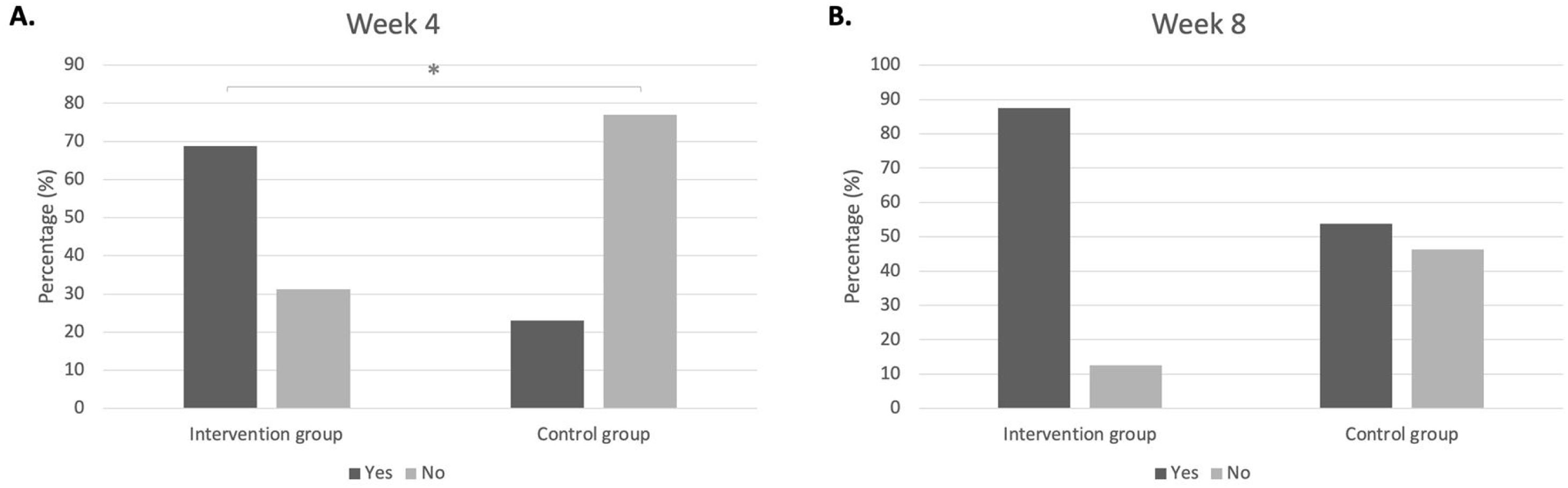
Clinical improvement was defined if the subjects fulfilled at least 2 from 3 criteria, which were VAS score < 5 (controlled), 1 degree reduction of the size of inferior turbinate, and improvement in PNIF value ≥ 20%. In week 4, intervention group has shown more clinical improvement compared to control group (p < 0.05). In week 8, there was no significant difference for clinical improvement in the intervention group compared to control group. Within the intervention group, clinical improvement before and after treatment was already found significant in week 4 (p < 0.001) yet was not significant within the control group (p > 0.05). In week 8, within both groups, the clinical improvement before and after treatment were found Fig. 2.
Clinical improvement in intervention group and control group, 4 weeks and 8 weeks after treatment. (A) Week 4 intervention group vs. control group; percentage of those who experience clinical improvement (68.8% vs. 23.1%); percentage of those who do not experience clinical improvement (31.2% vs. 76.9%), p = 0.014*). (B) Week 8 intervention group vs. control group; percentage of those who experience clinical improvement (87.5% vs. 53.8%); percentage of those who do not experience clinical improvement (12.5% vs. 46.2%), p = 0.092).
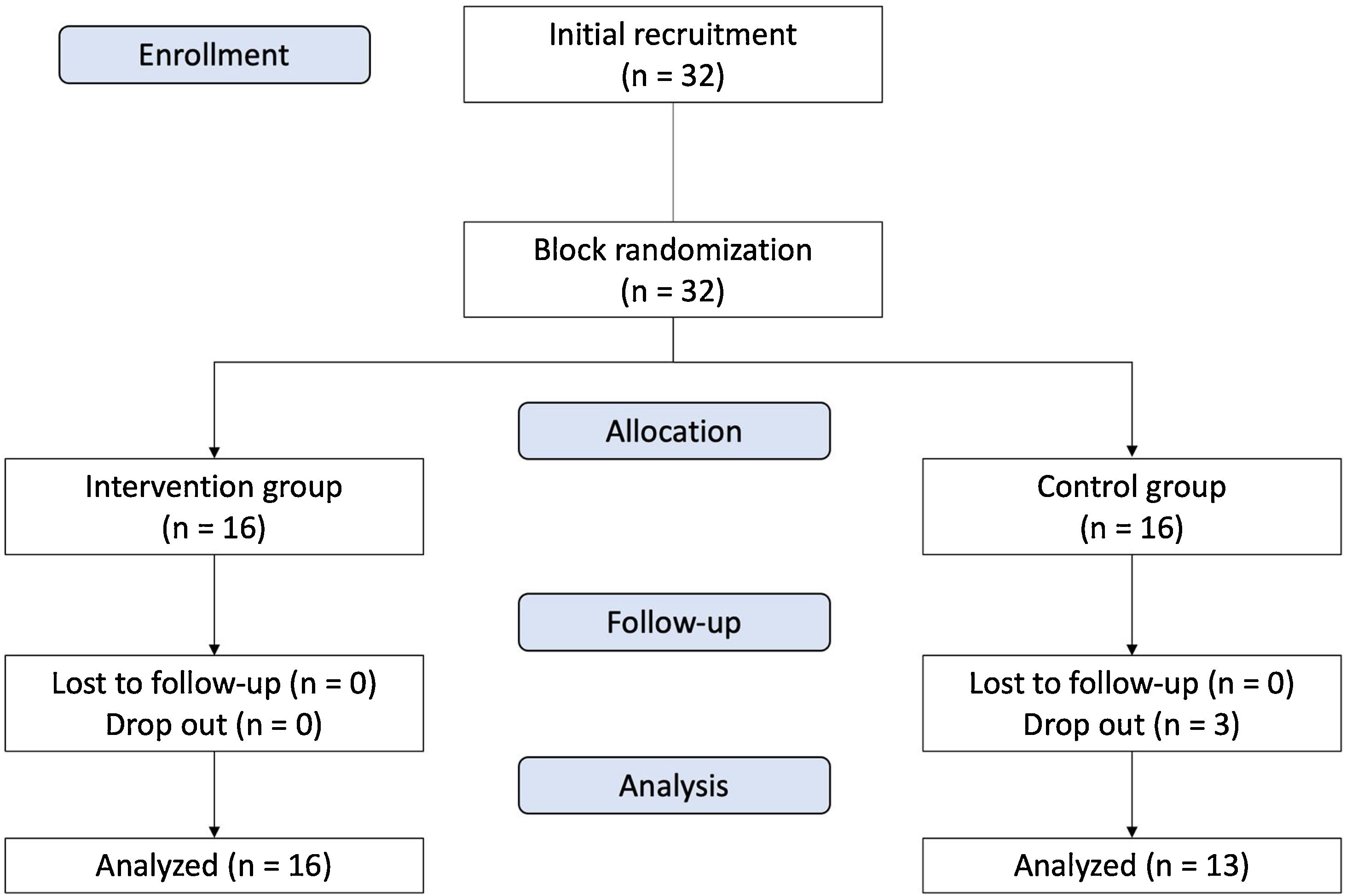
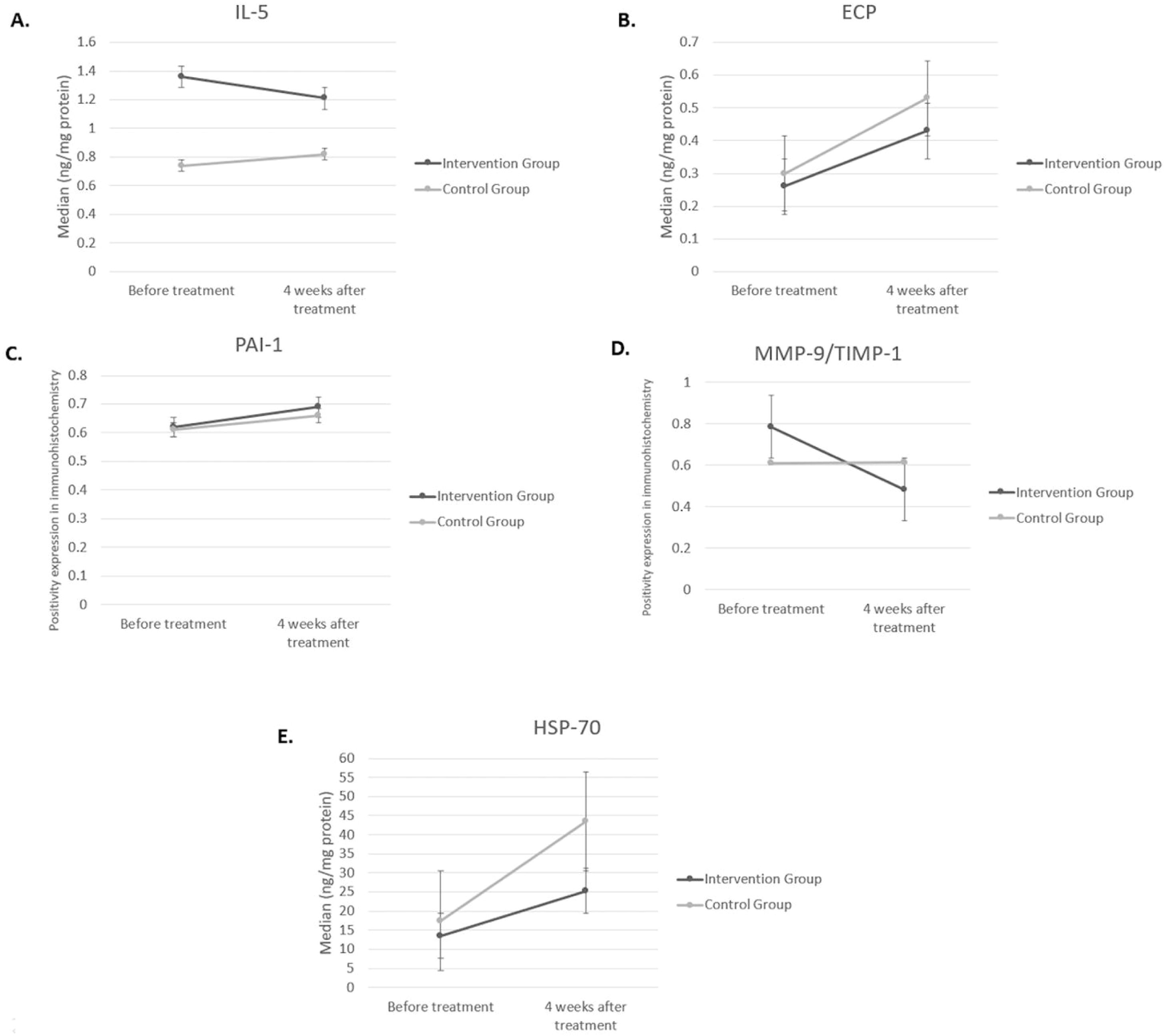
Statistical analysis of inflammatory and remodeling biomarkers can be seen in Fig. 3. Reduction in IL-5 was found in both groups, but the difference between both groups was not significant. Level of ECP was not changed in the intervention group, but a significant increase of ECP was found in the control group four weeks after treatment (p = 0.034). The difference of ECP level after treatment between the two groups was not significant (p > 0.05).
Biomolecular markers for inflammation (IL-5, ECP, HSP-70) and remodeling (PAI-1, MMP-9, and TIMP-1) in intervention group and control group, 4 weeks after treatment. (A) IL-5 (ng/mg) in intervention group vs. control group: before treatment (1.36 [1.04–1.91] vs. 0.74 [0.37–4.41], p = 0.511); 4 weeks after treatment (1.21 [0.82–1.62] vs. 0.82 [0.81–1.80], p = 0.965). (B) ECP (ng/mg) in intervention group vs. control group: before treatment (0.26 [0.21–0.60] vs. 0.30 [−0.12 to 1.56], p = 0.759); 4 weeks after treatment (0.43 [0.35–0.76] vs. 0.53 [0.41–1.10], p = 0.380). (C) PAI-1 (positivity expression) in intervention group vs. control group: before treatment (0.62 ± 0.14 vs. 0.61 ± 0.14, p = 0.972); 4 weeks after treatment (0.69 ± 0.17 vs. 0.66 ± 0.17, p = 0.714)). (D) MMP-9/TIMP-1 ratio (positivity expression) in intervention group vs. control group: before treatment (0.71 [0.67‒1.00] vs. 0.67 [0.33‒0.88], p = 0.104); 4 weeks after treatment (0.33 [0.25‒0.63] vs. 0.67 [0.50‒0.75], p = 0.035). (E) HSP-70 (ng/mg) in intervention group vs. control group: before treatment (13.47 [9.05–54.87] vs. 17.48 [.55–68.99], p = 0.726); 4 weeks after treatment (25.26 [13.21–78.21] vs. 43.51 (26.18–65.62), p = 0.188). IL-5, Interleukin-5; ECP, Eosinophil Cationic Protein; HSP-70, Heat Shock Protein-70; PAI-1, Plasminogen Activator Inhibitor Type 1; MMP-9, Matrix Metalloproteinase 9; TIMP-1, Tissue Inhibitor Metalloproteinase 1.
We assume that all subjects have the same baseline for the remodeling state of turbinate mucosa as we can see from the statistical analysis of MMP-9 and TIMP-1 ratio, which we found not statistically significant in baseline between the two groups. PAI-1 expression can be seen in Fig. 4. Increase of PAI-1 expression was found in both intervention and control group, there was no significant difference (p > 0.05). Reduction of MMP-9/TIMP-1 ratio was found more in intervention group compared to control group (p < 0.05). The expression of MMP-9 and TIMP-1 in both intervention and control group can be seen in Figs. 5 and 6, respectively.
Increase in HSP-70 level was found in both groups, but there was no significant difference (p > 0.05).
DiscussionBased on our statistical calculation, we achieved a minimum sample of 14 for each group to establish significance and allowing for a 10% drop-out rate. At the end of the study, we managed to achieve 16 subjects for each group. We aimed to collect more subjects if possible, however due to the pandemic situation in 2020, the recruitment for sample of the study was halted after the minimum sample was achieved as allergic rhinitis patients who seek for treatment to the policlinic were very limited. Further investigation with a larger sample size was needed to see whether our result was representable for the community.
In this study, clinical improvement was found significantly more in intervention group compared to control group in week 4 (RR = 2.36; 90% CI 1.10–5.07). In week 8, intervention group had more clinical improvement than control group, but the difference between the two groups were found not significant. These findings were in line with previous study by Gunhan et al. and Nease et al. which proved that radiofrequency turbinate reduction gave significant clinical improvement compared to control group.3,4 From this study, it could be seen that the control group had just responded to treatment and resulted in more clinical improvement in week 8 after treatment, whereas the intervention group had given significant clinical improvement in week 4. This suggests that early radiofrequency treatment helps fasten clinical improvement in moderate-severe persistent AR but did not alter the course of this chronic inflammatory disease. Therefore, maintenance therapy by pharmacotherapy should still be given to control inflammation.
A previous study by Ogawa et al. found a significant reduction in IL-5 and eotaxin proteins in patients who received turbinectomy.5 It was also confirmed by the histology finding that shown a reduction in inflammatory mediator cells in subepithelial mucosa. Salzano et al. found that after radiofrequency treatment, there will be a blood flow reduction to the inferior turbinate caused by necrosis and fibrosis in submucosa, and therefore reduce the number of inflammatory cells in the submucosal layer.6 Similar to those previous studies, in this study we also found a reduction of IL-5 level in intervention group compared to control group, but the difference between the two groups was not statistically significant.
In both groups, ECP level increased, but the difference between the groups was not statistically significant. Previous study by D’Amato et al. found that ECP level in seasonal AR is always fluctuating and given the immunotherapy will reduce the level of ECP significantly.7 On the other side, all of the subjects in this study were having perennial AR, therefore the ECP level tends to be steady at all times as the allergen exposure also happens over the year. Therefore, in AR perennial, the ECP level tends to not have a significant change even though treatment has been given. In further research, we suggest using other inflammatory markers to evaluate inflammation in persistent RA.
Both groups in this study shown an increase in PAI-1 expression in week 4 after treatment, yet the difference between the two groups was not statistically significant. A small increase in PAI-1 expression was still needed in order for fibrin deposition to occur and repair the epithelial barrier in AR, but according to Kim et al. and Milenkovic et al., a significant increase of PAI-1 would cause massive fibrin deposition in the alveoli of asthma patients and hence causing them to have severe exacerbation that could lead to death.8,9
In this study, we did not exclude patients regarding their turbinate state. We assume that all subjects have the same baseline for the remodeling state of turbinate mucosa as we can see from the statistical analysis of MMP-9 and TIMP-1 ratio, which we found not statistically significant in baseline between the two groups. A reduction of MMP-9/TIMP-1 ratio was found significantly more in the intervention group compared to control group. These findings were in lined with previous study by Kyo et al. that found a significant increase in TIMP-1 expression in the nasal mucosa after given topical corticosteroid. The high TIMP-1 will inhibit MMP-9 expression, therefore reduce the AR symptoms, and leads to physiological remodeling. Besides inhibiting MMP-9 expression, TIMP-1 also plays a role in reducing the migration of inflammatory cells by inhibiting ICAM-1 and VCAM-1 expression which was highly found in AR patients.10 Therefore, in this study we found early radiofrequency treatment might play a role in reducing pathological remodeling marker (MMP-9/TIMP-1 ratio) in moderate-severe persistent AR.
In this study, a rise in HSP-70 level was found in both the radiofrequency and control group, but the difference between the two groups was not statistically significant. A study by Min et al. found that HSP-70 level was high in nasal secretion of AR patients compared to non-AR patients.11 A previous study by Jones et al. found that hyperthermic treatment will stimulate HSP-70 synthesis and this high level of HSP-70 was found to have a role as anti-apoptosis and increase cell survival.12 Also, after given hyperthermic treatment, cell will have an acquired tolerance to the upcoming stressors. This protection acquired by the hyperthermic treatment also have an anti-inflammatory component. This was also supported by Zininga et al. who stated that a high level of HSP-70 will stimulate the production of anti-inflammatory cytokine IL-10 to keep the homeostasis of HSP-70.13
Based on the Allergic Rhinitis and Its Impact on Asthma (ARIA) guideline established by World Health Organization (WHO) 2008, pharmacotherapy for persistent moderate-severe RA was given for 2–4 weeks and if the symptoms improved, treatment should be continued for one month. Referring to the guideline and adjusting for subjects’ compliance, we chose the minimum period for follow-up, which is 8 weeks, to see the outcome of the treatment. We realize, further investigation was needed with a longer period of follow-up to see the long-term effect of radiofrequency in persistent moderate-severe RA.
LimitationWe realized, our limited sample size and short period of follow-up were our study limitation. With a larger sample size, we might obtain a higher statistical power among the result of inflammatory markers. Therefore, further investigation with a larger sample size and longer period of follow-up were needed to see the applicability of our result in the community and long-term effect of radiofrequency. Besides, HSP-70 was not evaluated directly after radiofrequency turbinate reduction treatment, thus we could not evaluate the direct effect of thermal in radiofrequency.
ConclusionEarly radiofrequency turbinate reduction followed by pharmacotherapy given to persistent moderate-severe AR patients give more improvement only in early clinical symptoms and reduce MMP-9/TIMP-1 ratio, thus it can be suggested as one of the adjuvant therapies for the management of moderate-severe persistent AR. However, further investigation with a larger sample size and longer follow-up period is needed.
Ethical approvalAll procedures performed in this study involving human participants were in concordance with the ethical standards of the local ethics committee.
Conflicts of interestThe authors declare no conflicts of interest.
This research was supported by the Universitas Indonesia Grant PIT9 in 2019 with Contract Number: NKB-0117/UN2.R3.1/HKP.05.00/2019.
Peer Review under the responsibility of Associação Brasileira de Otorrinolaringologia e Cirurgia Cérvico-Facial.




![Biomolecular markers for inflammation (IL-5, ECP, HSP-70) and remodeling (PAI-1, MMP-9, and TIMP-1) in intervention group and control group, 4 weeks after treatment. (A) IL-5 (ng/mg) in intervention group vs. control group: before treatment (1.36 [1.04–1.91] vs. 0.74 [0.37–4.41], p = 0.511); 4 weeks after treatment (1.21 [0.82–1.62] vs. 0.82 [0.81–1.80], p = 0.965). (B) ECP (ng/mg) in intervention group vs. control group: before treatment (0.26 [0.21–0.60] vs. 0.30 [−0.12 to 1.56], p = 0.759); 4 weeks after treatment (0.43 [0.35–0.76] vs. 0.53 [0.41–1.10], p = 0.380). (C) PAI-1 (positivity expression) in intervention group vs. control group: before treatment (0.62 ± 0.14 vs. 0.61 ± 0.14, p = 0.972); 4 weeks after treatment (0.69 ± 0.17 vs. 0.66 ± 0.17, p = 0.714)). (D) MMP-9/TIMP-1 ratio (positivity expression) in intervention group vs. control group: before treatment (0.71 [0.67‒1.00] vs. 0.67 [0.33‒0.88], p = 0.104); 4 weeks after treatment (0.33 [0.25‒0.63] vs. 0.67 [0.50‒0.75], p = 0.035). (E) HSP-70 (ng/mg) in intervention group vs. control group: before treatment (13.47 [9.05–54.87] vs. 17.48 [.55–68.99], p = 0.726); 4 weeks after treatment (25.26 [13.21–78.21] vs. 43.51 (26.18–65.62), p = 0.188). IL-5, Interleukin-5; ECP, Eosinophil Cationic Protein; HSP-70, Heat Shock Protein-70; PAI-1, Plasminogen Activator Inhibitor Type 1; MMP-9, Matrix Metalloproteinase 9; TIMP-1, Tissue Inhibitor Metalloproteinase 1. Biomolecular markers for inflammation (IL-5, ECP, HSP-70) and remodeling (PAI-1, MMP-9, and TIMP-1) in intervention group and control group, 4 weeks after treatment. (A) IL-5 (ng/mg) in intervention group vs. control group: before treatment (1.36 [1.04–1.91] vs. 0.74 [0.37–4.41], p = 0.511); 4 weeks after treatment (1.21 [0.82–1.62] vs. 0.82 [0.81–1.80], p = 0.965). (B) ECP (ng/mg) in intervention group vs. control group: before treatment (0.26 [0.21–0.60] vs. 0.30 [−0.12 to 1.56], p = 0.759); 4 weeks after treatment (0.43 [0.35–0.76] vs. 0.53 [0.41–1.10], p = 0.380). (C) PAI-1 (positivity expression) in intervention group vs. control group: before treatment (0.62 ± 0.14 vs. 0.61 ± 0.14, p = 0.972); 4 weeks after treatment (0.69 ± 0.17 vs. 0.66 ± 0.17, p = 0.714)). (D) MMP-9/TIMP-1 ratio (positivity expression) in intervention group vs. control group: before treatment (0.71 [0.67‒1.00] vs. 0.67 [0.33‒0.88], p = 0.104); 4 weeks after treatment (0.33 [0.25‒0.63] vs. 0.67 [0.50‒0.75], p = 0.035). (E) HSP-70 (ng/mg) in intervention group vs. control group: before treatment (13.47 [9.05–54.87] vs. 17.48 [.55–68.99], p = 0.726); 4 weeks after treatment (25.26 [13.21–78.21] vs. 43.51 (26.18–65.62), p = 0.188). IL-5, Interleukin-5; ECP, Eosinophil Cationic Protein; HSP-70, Heat Shock Protein-70; PAI-1, Plasminogen Activator Inhibitor Type 1; MMP-9, Matrix Metalloproteinase 9; TIMP-1, Tissue Inhibitor Metalloproteinase 1.](https://static.elsevier.es/multimedia/18088694/0000008900000002/v1_202303281144/S1808869422000416/v1_202303281144/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/7hLX2FbBoxC1192i158SI=)